Related Research Articles

Vancomycin is a glycopeptide antibiotic medication used to treat a number of bacterial infections. It is used intravenously as a treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis caused by methicillin-resistant Staphylococcus aureus. Blood levels may be measured to determine the correct dose. Vancomycin is also taken orally as a treatment for severe Clostridium difficile colitis. When taken orally it is poorly absorbed.

Polymyxins are antibiotics. Polymyxins B and E are used in the treatment of Gram-negative bacterial infections. They work mostly by breaking up the bacterial cell membrane. They are part of a broader class of molecules called nonribosomal peptides.

Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune signaling activities, or both. They are variously active against bacteria, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

Tyrocidine is a mixture of cyclic decapeptides produced by the bacteria Bacillus brevis found in soil. It can be composed of 4 different amino acid sequences, giving tyrocidine A–D. Tyrocidine is the major constituent of tyrothricin, which also contains gramicidin. Tyrocidine was the first commercially available antibiotic, but has been found to be toxic toward human blood and reproductive cells. The function of tyrocidine within its host B. brevis is thought to be regulation of sporulation.

Surfactin is a cyclic lipopeptide, commonly used as an antibiotic for its capacity as a surfactant. It is an amphiphile capable of withstanding hydrophilic and hydrophobic environments. The Gram-positive bacterial species Bacillus subtilis produces surfactin for its antibiotic effects against competitors. Surfactin showcases antibacterial, antiviral, antifungal, and hemolytic effects.

Cathelicidin antimicrobial peptide (CAMP) is a polypeptide that is primarily stored in the lysosomes of macrophages and polymorphonuclear leukocytes (PMNs); in humans, the CAMP gene encodes the peptide precursor CAP-18, which is processed by proteinase 3-mediated extracellular cleavage into the active form LL-37. LL-37 is the only peptide in the Cathelicidin family found in the human body.
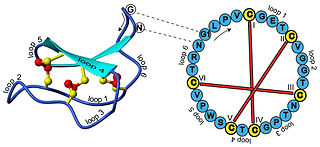
In biochemistry, cyclotides are small, disulfide-rich peptides isolated from plants. Typically containing 28-37 amino acids, they are characterized by their head-to-tail cyclised peptide backbone and the interlocking arrangement of their three disulfide bonds. These combined features have been termed the cyclic cystine knot (CCK) motif. To date, over 100 cyclotides have been isolated and characterized from species of the families Rubiaceae, Violaceae, and Cucurbitaceae. Cyclotides have also been identified in agriculturally important families such as the Fabaceae and Poaceae.

Alpha defensins are a family of mammalian defensin peptides of the alpha subfamily. In mammals they are also known as cryptdins and are produced within the small bowel. Cryptdin is a portmanteau of crypt and defensin.
Beta defensins are a family of vertebrate defensins. The beta defensins are antimicrobial peptides implicated in the resistance of epithelial surfaces to microbial colonization.

Class II bacteriocins are a class of small peptides that inhibit the growth of various bacteria.
Pseudin is a peptide derived from Pseudis paradoxa. Pseudins have some antimicrobial function.
Polymers with the ability to kill or inhibit the growth of microorganisms such as bacteria, fungi, or viruses are classified as antimicrobial agents. This class of polymers consists of natural polymers with inherent antimicrobial activity and polymers modified to exhibit antimicrobial activity. Polymers are generally nonvolatile, chemically stable, and can be chemically and physically modified to display desired characteristics and antimicrobial activity. Antimicrobial polymers are a prime candidate for use in the food industry to prevent bacterial contamination and in water sanitation to inhibit the growth of microorganisms in drinking water.
Cephalosporins are a broad class of bactericidal antibiotics that include the β-lactam ring and share a structural similarity and mechanism of action with other β-lactam antibiotics. The cephalosporins have the ability to kill bacteria by inhibiting essential steps in the bacterial cell wall synthesis which in the end results in osmotic lysis and death of the bacterial cell. Cephalosporins are widely used antibiotics because of their clinical efficiency and desirable safety profile.
Teixobactin is a peptide-like secondary metabolite of some species of bacteria, that kills some gram-positive bacteria. It appears to belong to a new class of antibiotics, and harms bacteria by binding to lipid II and lipid III, important precursor molecules for forming the cell wall.
Esculentin-2CHa is an antimicrobial peptide located outside the epithelial cell's membrane of the skin of many species of amphibians, such as Rana chiricahuensis. This peptide has recently become more important due to its defense response function and its possible application in the treatment of various human pathologies, that range from type 2 diabetes to bacterial and fungi infections. Esculentin-2CHa is a peptide that belongs to the Esculentin-2 family, which is known for its broad-spectrum of antimicrobial activity and its low cytotoxicity to human erythrocytes. However, not much is known about its structures and their relation to the functions these peptides carry out.
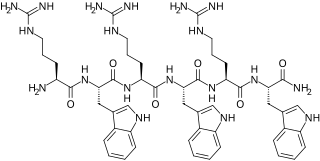
MP196 is a synthetic antimicrobial peptide. It falls under the structural class: short cationic peptides. Since it is a short cationic peptide, it can be easily synthesized, derivatized and isolated. MP196 is rich in tryptophan, a hydrophobic amino acid and arginine residues, a positively charged amino acid. It has structure: RWRWRW-NH2. This a short linear peptide with minimal pharmacophore. MP196 is effective against gram-positive bacteria and moderately effective against gram-negative bacteria.

Robert Ernest William Hancock is a Canadian microbiologist and University of British Columbia Killam Professor of Microbiology and Immunology, an Associate Faculty Member of the Wellcome Trust Sanger Institute, and a Canada Research Chair in Health and Genomics.

Murepavadin also known as POL7080 is a Pseudomonas specific peptidomimetic antibiotic. It is a synthetic cyclic beta hairpin peptidomimetic based on the cationic antimicrobial peptide protegrin I (PG-1) and the first example of an outer membrane protein-targeting antibiotic class with a novel, nonlytic mechanism of action, highly active and selective against the protein transporter LptD of Pseudomonas aeruginosa. In preclinical studies the compound was highly active on a broad panel of clinical isolates including multi-drug resistant Pseudomonas bacteria with outstanding in vivo efficacy in sepsis, lung, and thigh infection models. Intravenous murepavadin is in development for the treatment of bacterial hospital-acquired pneumonia and bacterial ventilator-associated pneumonia due to Pseudomonas aeruginosa.
A proteolipid is a protein covalently linked to lipid molecules, which can be fatty acids, isoprenoids or sterols. The process of such a linkage is known as protein lipidation, and falls into the wider category of acylation and post-translational modification. Proteolipids are abundant in brain tissue, and are also present in many other animal and plant tissues. They include ghrelin, a peptide hormone associated with feeding. Many proteolipids are composed of proteins covalenently bound to fatty acid chains, often granting them an interface for interacting with biological membranes. They are not to be confused with lipoproteins, a kind of spherical assembly made up of many molecules of lipids and some apolipoproteins.
References
- ↑ Amiche M, Seon AA, Pierre TN, Nicolas P (August 1999). "The dermaseptin precursors: a protein family with a common preproregion and a variable C-terminal antimicrobial domain". FEBS Letters. 456 (3): 352–6. doi: 10.1016/s0014-5793(99)00964-3 . PMID 10462042.
- ↑ Hancock RE, Falla T, Brown M (1995). "Cationic bactericidal peptides". Advances in Microbial Physiology. 37: 135–75. doi:10.1016/s0065-2911(08)60145-9. ISBN 9780120277377. PMID 8540420.
- ↑ Amiche M, Ducancel F, Mor A, Boulain JC, Menez A, Nicolas P (July 1994). "Precursors of vertebrate peptide antibiotics dermaseptin b and adenoregulin have extensive sequence identities with precursors of opioid peptides dermorphin, dermenkephalin, and deltorphins". The Journal of Biological Chemistry. 269 (27): 17847–52. doi: 10.1016/S0021-9258(17)32386-4 . PMID 8074751.
- ↑ Mor A (2000). "Peptide-based antibiotics: a potential answer to raging antimicrobial resistance". Drug Development Research. 50 (34): 440–447. doi:10.1002/1098-2299(200007/08)50:3/4<440::aid-ddr27>3.3.co;2-w.
- ↑ Feder R, Nehushtai R, Mor A (October 2001). "Affinity driven molecular transfer from erythrocyte membrane to target cells". Peptides. 22 (10): 1683–90. doi:10.1016/s0196-9781(01)00504-6. PMID 11587797. S2CID 699138.