Related Research Articles
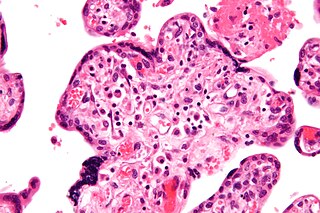
Intrauterine growth restriction (IUGR), or fetal growth restriction, is the poor growth of a fetus while in the womb during pregnancy. IUGR is defined by clinical features of malnutrition and evidence of reduced growth regardless of an infant's birth weight percentile. The causes of IUGR are broad and may involve maternal, fetal, or placental complications.
A maternal effect is a situation where the phenotype of an organism is determined not only by the environment it experiences and its genotype, but also by the environment and genotype of its mother. In genetics, maternal effects occur when an organism shows the phenotype expected from the genotype of the mother, irrespective of its own genotype, often due to the mother supplying messenger RNA or proteins to the egg. Maternal effects can also be caused by the maternal environment independent of genotype, sometimes controlling the size, sex, or behaviour of the offspring. These adaptive maternal effects lead to phenotypes of offspring that increase their fitness. Further, it introduces the concept of phenotypic plasticity, an important evolutionary concept. It has been proposed that maternal effects are important for the evolution of adaptive responses to environmental heterogeneity.
Thrifty phenotype refers to the correlation between low birth weight of neonates and the increased risk of developing metabolic syndromes later in life, including type 2 diabetes and cardiovascular diseases. Although early life undernutrition is thought to be the key driving factor to the hypothesis, other environmental factors have been explored for their role in susceptibility, such as physical inactivity. Genes may also play a role in susceptibility of these diseases, as they may make individuals predisposed to factors that lead to increased disease risk.
Prenatal development includes the development of the embryo and of the fetus during a viviparous animal's gestation. Prenatal development starts with fertilization, in the germinal stage of embryonic development, and continues in fetal development until birth.

The causes of autism are environmental or genetic factors that predispose an individual to develop autism, also known as autism spectrum disorder (ASD). Many causes of autism have been proposed, but understanding of the theory of causation of autism is incomplete. Attempts have been made to incorporate the known genetic and environmental causes into a comprehensive causative framework. ASD is a neurodevelopmental disorder marked by impairments in communicative ability and social interaction and restricted/repetitive behaviors, interests, or activities not suitable for the individual's developmental stage. The severity of symptoms and functional impairment vary between individuals.

Protein–energy undernutrition (PEU), once called protein-energy malnutrition (PEM), is a form of malnutrition that is defined as a range of conditions arising from coincident lack of dietary protein and/or energy (calories) in varying proportions. The condition has mild, moderate, and severe degrees.
Metabolic imprinting refers to the long-term physiological and metabolic effects that an offspring's prenatal and postnatal environments have on them. Perinatal nutrition has been identified as a significant factor in determining an offspring's likelihood of it being predisposed to developing cardiovascular disease, obesity, and type 2 diabetes amongst other conditions.
Prenatal stress is exposure of an expectant mother to psychosocial or physical stress, which can be caused by daily life events or by environmental hardships. This psychosocial or physical stress that the expectant mother is experiencing has an effect on the fetus. According to the Developmental Origins of Health and Disease (DOHaD), a wide range of environmental factors a woman may experience during the perinatal period can contribute to biological impacts and changes in the fetus that then causes health risks later in the child's life.

Prenatal nutrition addresses nutrient recommendations before and during pregnancy. Nutrition and weight management before and during pregnancy has a profound effect on the development of infants. This is a rather critical time for healthy development since infants rely heavily on maternal stores and nutrient for optimal growth and health outcome later in life.
Nutriepigenomics is the study of food nutrients and their effects on human health through epigenetic modifications. There is now considerable evidence that nutritional imbalances during gestation and lactation are linked to non-communicable diseases, such as obesity, cardiovascular disease, diabetes, hypertension, and cancer. If metabolic disturbances occur during critical time windows of development, the resulting epigenetic alterations can lead to permanent changes in tissue and organ structure or function and predispose individuals to disease.
Autism spectrum disorder (ASD) refers to a variety of conditions typically identified by challenges with social skills, communication, speech, and repetitive sensory-motor behaviors. The 11th International Classification of Diseases (ICD-11), released in January 2021, characterizes ASD by the associated deficits in the ability to initiate and sustain two-way social communication and restricted or repetitive behavior unusual for the individual's age or situation. Although linked with early childhood, the symptoms can appear later as well. Symptoms can be detected before the age of two and experienced practitioners can give a reliable diagnosis by that age. However, official diagnosis may not occur until much older, even well into adulthood. There is a large degree of variation in how much support a person with ASD needs in day-to-day life. This can be classified by a further diagnosis of ASD level 1, level 2, or level 3. Of these, ASD level 3 describes people requiring very substantial support and who experience more severe symptoms. ASD-related deficits in nonverbal and verbal social skills can result in impediments in personal, family, social, educational, and occupational situations. This disorder tends to have a strong correlation with genetics along with other factors. More research is identifying ways in which epigenetics is linked to autism. Epigenetics generally refers to the ways in which chromatin structure is altered to affect gene expression. Mechanisms such as cytosine regulation and post-translational modifications of histones. Of the 215 genes contributing, to some extent in ASD, 42 have been found to be involved in epigenetic modification of gene expression. Some examples of ASD signs are specific or repeated behaviors, enhanced sensitivity to materials, being upset by changes in routine, appearing to show reduced interest in others, avoiding eye contact and limitations in social situations, as well as verbal communication. When social interaction becomes more important, some whose condition might have been overlooked suffer social and other exclusion and are more likely to have coexisting mental and physical conditions. Long-term problems include difficulties in daily living such as managing schedules, hypersensitivities, initiating and sustaining relationships, and maintaining jobs.
The epigenetics of schizophrenia is the study of how inherited epigenetic changes are regulated and modified by the environment and external factors and how these changes influence the onset and development of, and vulnerability to, schizophrenia. Epigenetics concerns the heritability of those changes, too. Schizophrenia is a debilitating and often misunderstood disorder that affects up to 1% of the world's population. Although schizophrenia is a heavily studied disorder, it has remained largely impervious to scientific understanding; epigenetics offers a new avenue for research, understanding, and treatment.
A predictive adaptive response (PAR) is a developmental trajectory taken by an organism during a period of developmental plasticity in response to perceived environmental cues. This PAR does not confer an immediate advantage to the developing organism; however, if the PAR correctly anticipates the postnatal environment it will be advantageous in later life, if the environment the organism is born into differs from that anticipated by the PAR it will result in a mismatch. PAR mechanisms were first recognized in research done on human fetuses that investigated whether poor nutrition results in the inevitable diagnosis of Type 2 diabetes in later life. PARs are thought to occur through epigenetic mechanisms that alter gene expression, such as DNA methylation and histone modification, and do not involve changes to the DNA sequence of the developing organism. Examples of PARs include greater helmet development in Daphnia cucullata in response to maternal exposure to predator pheromones, rats exposed to glucocorticoid during late gestation led to an intolerance to glucose as adults, and coat thickness determination in vole pups by the photoperiod length experienced by the mother. Two hypotheses to explain PAR are the "thrifty phenotype" hypothesis and the developmental plasticity hypothesis.
The fetal origins hypothesis proposes that the period of gestation has significant impacts on the developmental health and wellbeing outcomes for an individual ranging from infancy to adulthood. The effects of fetal origin are marked by three characteristics: latency, wherein effects may not be apparent until much later in life; persistency, whereby conditions resulting from a fetal effect continue to exist for a given individual; and genetic programming, which describes the 'switching on' of a specific gene due to prenatal environment. Research in the areas of economics, epidemiology, and epigenetics offer support for the hypothesis.
Maternal fetal stress transfer is a physiological phenomenon in which psychosocial stress experienced by a mother during her pregnancy can be transferred to the fetus. Psychosocial stress describes the brain's physiological response to perceived social threat. Because of a link in blood supply between a mother and fetus, it has been found that stress can leave lasting effects on a developing fetus, even before a child is born. According to recent studies, these effects are mainly the result of two particular stress biomarkers circulating in the maternal blood supply: cortisol and catecholamines.
Fetal programming, also known as prenatal programming, is the theory that environmental cues experienced during fetal development play a seminal role in determining health trajectories across the lifespan.
Gene-environment interplay is a term encompassing multiple ways that genes and environments work together to produce a phenotype, or observable trait. Processes classified as examples of gene-environment interplay include gene-environment interaction, gene-environment correlation, and epigenetics, which is the study of the effect of the environment on gene expression. It is often studied with behavioral genetic research designs like twin, family, and adoption studies.
The first 1,000 days describes the period from conception to 24 months of age in child development. This is considered a "critical period" in which sufficient nutrition and environmental factors have life-long effects on a child's overall health. While adequate nutrition can be exceptionally beneficial during this critical period, inadequate nutrition may also be detrimental to the child. This is because children establish many of their lifetime epigenetic characteristics in their first 1,000 days. Medical and public health interventions early on in child development during the first 1,000 days may have higher rates of success compared to those achieved outside of this period.
Kent L. Thornburg is an American scientist, researcher and professor. He lives in Portland, Oregon and works at Oregon Health & Science University (OHSU), in the School of Medicine. He is the director for both the OHSU Center for Developmental Health and the Moore Institute for Nutrition & Wellness
Nutritional epigenetics is a science that studies the effects of nutrition on gene expression and chromatin accessibility. It is a subcategory of nutritional genomics that focuses on the effects of bioactive food components on epigenetic events.
References
- 1 2 3 Wadhwa PD, Buss C, Entringer S, Swanson JM (September 2009). "Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms". Seminars in Reproductive Medicine. 27 (5): 358–368. doi:10.1055/s-0029-1237424. PMC 2862635 . PMID 19711246.
- ↑ Gillman MW (October 2005). "Developmental origins of health and disease". The New England Journal of Medicine. 353 (17): 1848–1850. doi:10.1056/NEJMe058187. PMC 1488726 . PMID 16251542.
- ↑ Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA (May 2007). "Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease". Pediatric Research. 61 (5 Pt 2): 5R–10R. doi: 10.1203/pdr.0b013e318045bedb . PMID 17413851.
- ↑ Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, et al. (October 2015). "Developmental Origins of Health and Disease: Integrating Environmental Influences". Endocrinology. 156 (10): 3416–3421. doi:10.1210/EN.2015-1394. PMC 4588819 . PMID 26241070.
- ↑ O'Donnell KJ, Meaney MJ (April 2017). "Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis". The American Journal of Psychiatry. 174 (4): 319–328. doi: 10.1176/appi.ajp.2016.16020138 . PMID 27838934.
- 1 2 3 4 Rosenfeld CS, ed. (2016). The Epigenome and Developmental Origins of Health and Disease. doi:10.1016/c2013-0-23131-7. ISBN 978-0-12-801383-0.
- 1 2 Arima Y, Fukuoka H (July 2020). "Developmental origins of health and disease theory in cardiology". Journal of Cardiology. 76 (1): 14–17. doi: 10.1016/j.jjcc.2020.02.003 . PMID 32115330. S2CID 211726894.
- ↑ "What is Epigenetics?". Centers for Disease Control and Prevention. 2022-08-15. Retrieved 2023-02-06.
- ↑ Almond D, Currie J (2011-08-01). "Killing Me Softly: The Fetal Origins Hypothesis". The Journal of Economic Perspectives. 25 (3): 153–172. doi:10.1257/jep.25.3.153. PMC 4140221 . PMID 25152565.
- ↑ Henriksen T, Clausen T (February 2002). "The fetal origins hypothesis: placental insufficiency and inheritance versus maternal malnutrition in well-nourished populations". Acta Obstetricia et Gynecologica Scandinavica. 81 (2): 112–114. doi:10.1034/j.1600-0412.2002.810204.x. PMID 11942899. S2CID 25255975.
- 1 2 Schulz LC (September 2010). "The Dutch Hunger Winter and the developmental origins of health and disease". Proceedings of the National Academy of Sciences of the United States of America. 107 (39): 16757–16758. Bibcode:2010PNAS..10716757S. doi: 10.1073/pnas.1012911107 . PMC 2947916 . PMID 20855592.
- ↑ Hales CN, Barker DJ (July 1992). "Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis". Diabetologia. 35 (7): 595–601. doi: 10.1007/BF00400248 . PMID 1644236. S2CID 35676219.
- 1 2 Roseboom T, de Rooij S, Painter R (August 2006). "The Dutch famine and its long-term consequences for adult health". Early Human Development. Special Abstract Issue. 82 (8): 485–491. doi:10.1016/j.earlhumdev.2006.07.001. PMID 16876341.
- ↑ Hales CN, Barker DJ (2001-11-01). "The thrifty phenotype hypothesis". British Medical Bulletin. 60 (1): 5–20. doi: 10.1093/bmb/60.1.5 . PMID 11809615.
- 1 2 3 Gilbert SF, Epel D (2009). Ecological developmental biology : integrating epigenetics, medicine, and evolution. Sinauer Associates. ISBN 978-0-87893-299-3. OCLC 909806681.
- 1 2 3 4 5 6 7 Goyal D, Limesand SW, Goyal R (July 2019). "Epigenetic responses and the developmental origins of health and disease". The Journal of Endocrinology. 242 (1): T105–T119. doi: 10.1530/JOE-19-0009 . PMID 31091503. S2CID 155101302.
- ↑ Grote P, Wittler L, Hendrix D, Koch F, Währisch S, Beisaw A, et al. (January 2013). "The tissue-specific lncRNA Fendrr is an essential regulator of heart and body wall development in the mouse". Developmental Cell. 24 (2): 206–214. doi:10.1016/j.devcel.2012.12.012. PMC 4149175 . PMID 23369715.
- ↑ Entringer S, Buss C, Wadhwa PD (December 2010). "Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings". Current Opinion in Endocrinology, Diabetes, and Obesity. 17 (6): 507–516. doi:10.1097/MED.0b013e3283405921. PMC 3124255 . PMID 20962631.
- ↑ Arenz S, Rückerl R, Koletzko B, von Kries R (October 2004). "Breast-feeding and childhood obesity--a systematic review". International Journal of Obesity and Related Metabolic Disorders. 28 (10): 1247–1256. doi: 10.1038/sj.ijo.0802758 . PMID 15314625. S2CID 25205202.
- ↑ Godfrey KM, Forrester T, Barker DJ, Jackson AA, Landman JP, Hall JS, et al. (May 1994). "Maternal nutritional status in pregnancy and blood pressure in childhood". British Journal of Obstetrics and Gynaecology. 101 (5): 398–403. doi:10.1111/j.1471-0528.1994.tb11911.x. PMID 8018610. S2CID 3102246.
- ↑ Ravelli GP, Stein ZA, Susser MW (August 1976). "Obesity in young men after famine exposure in utero and early infancy". The New England Journal of Medicine. 295 (7): 349–353. doi:10.1056/NEJM197608122950701. PMID 934222.
- ↑ Kereliuk SM, Brawerman GM, Dolinsky VW (July 2017). "Maternal Macronutrient Consumption and the Developmental Origins of Metabolic Disease in the Offspring". International Journal of Molecular Sciences. 18 (7): 1451. doi: 10.3390/ijms18071451 . PMC 5535942 . PMID 28684678.
- ↑ "Autism spectrum disorder Information". Mount Sinai Health System. New York. Retrieved 2023-04-13.
- 1 2 Xu MQ, Sun WS, Liu BX, Feng GY, Yu L, Yang L, et al. (May 2009). "Prenatal malnutrition and adult schizophrenia: further evidence from the 1959-1961 Chinese famine". Schizophrenia Bulletin. 35 (3): 568–576. doi:10.1093/schbul/sbn168. PMC 2669578 . PMID 19155344.
- 1 2 He P, Chen G, Guo C, Wen X, Song X, Zheng X (June 2018). "Long-term effect of prenatal exposure to malnutrition on risk of schizophrenia in adulthood: Evidence from the Chinese famine of 1959-1961". European Psychiatry. 51: 42–47. doi:10.1016/j.eurpsy.2018.01.003. PMID 29514118. S2CID 3804975.
- ↑ Hoek HW, Brown AS, Susser E (August 1998). "The Dutch famine and schizophrenia spectrum disorders". Social Psychiatry and Psychiatric Epidemiology. 33 (8): 373–379. doi:10.1007/s001270050068. PMID 9708024. S2CID 30257704.
- 1 2 3 4 Brown AS, Susser ES (November 2008). "Prenatal nutritional deficiency and risk of adult schizophrenia". Schizophrenia Bulletin. 34 (6): 1054–1063. doi:10.1093/schbul/sbn096. PMC 2632499 . PMID 18682377.
- ↑ McGrath J, Brown A, St Clair D (March 2011). "Prevention and schizophrenia--the role of dietary factors". Schizophrenia Bulletin. 37 (2): 272–283. doi:10.1093/schbul/sbq121. PMC 3044637 . PMID 20974747.
- ↑ Dolinoy DC, Das R, Weidman JR, Jirtle RL (May 2007). "Metastable epialleles, imprinting, and the fetal origins of adult diseases". Pediatric Research. 61 (5 Pt 2): 30R–37R. doi: 10.1203/pdr.0b013e31804575f7 . PMID 17413847.
- ↑ Insel BJ, Schaefer CA, McKeague IW, Susser ES, Brown AS (October 2008). "Maternal iron deficiency and the risk of schizophrenia in offspring". Archives of General Psychiatry. 65 (10): 1136–1144. doi:10.1001/archpsyc.65.10.1136. PMC 3656467 . PMID 18838630.
- ↑ Youdim MB, Ben-Shachar D, Ashkenazi R, Yehuda S (1983). "Brain iron and dopamine receptor function". Advances in Biochemical Psychopharmacology. 37: 309–321. PMID 6138953.
- 1 2 3 Coussons-Read ME (June 2013). "Effects of prenatal stress on pregnancy and human development: mechanisms and pathways". Obstetric Medicine. 6 (2): 52–57. doi:10.1177/1753495X12473751. PMC 5052760 . PMID 27757157.
- 1 2 3 "Maternal Stress During Pregnancy Linked to Infant Illness | UC San Francisco". www.ucsf.edu. Retrieved 2023-04-19.