
Aromatic compounds or arenes usually refers to organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's Rule. Aromatic compounds have the following general properties:
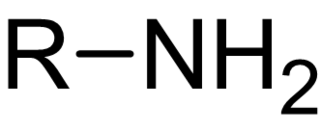
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Formally, amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.
In chemistry, a pentose is a monosaccharide with five carbon atoms. The chemical formula of many pentoses is C
5H
10O
5, and their molecular weight is 150.13 g/mol.

Acridine is an organic compound and a nitrogen heterocycle with the formula C13H9N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecules pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid, which crystallizes in needles. There are few commercial applications of acridines; at one time acridine dyes were popular, but they are now relegated to niche applications, such as with acridine orange. The name is a reference to the acrid odour and acrid skin-irritating effect of the compound.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.

1,8-Bis(dimethylamino)naphthalene is an organic compound with the formula C10H6(NMe2)2 (Me = methyl). It is classified as a peri-naphthalene, i.e. a 1,8-disubstituted derivative of naphthalene. Owing to its unusual structure, it exhibits exceptional basicity. It is often referred by the trade name Proton Sponge, a trademark of Sigma-Aldrich.

Triazines are a class of nitrogen-containing heterocycles. The parent molecules' molecular formula is C3H3N3. They exist in three isomeric forms, 1,3,5-triazines being common.
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction.
In stereochemistry, topicity is the stereochemical relationship between substituents and the structure to which they are attached. Depending on the relationship, such groups can be heterotopic, homotopic, enantiotopic, or diastereotopic.
The Combes quinoline synthesis is a chemical reaction, which was first reported by Combes in 1888. Further studies and reviews of the Combes quinoline synthesis and its variations have been published by Alyamkina et al., Bergstrom and Franklin, Born, and Johnson and Mathews.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.

The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids.
These drugs are known in the UK as controlled drug, because this is the term by which the act itself refers to them. In more general terms, however, many of these drugs are also controlled by the Medicines Act 1968, there are many other drugs which are controlled by the Medicines Act but not by the Misuse of Drugs Act, and some other drugs are controlled by other laws.

Benzoxazines are a group of isomeric bicyclic heterocyclic chemical compounds that consist of a benzene ring fused to an oxazine ring. The different isomers depend on the relative positions of the oxygen and nitrogen atoms in the oxazine ring, on the location of ring fusion, and on the position of the double bond in the oxazine ring. They have the molecular formula C8H7NO.
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic system is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, alkylation Friedel–Crafts reaction and acylation Friedel–Crafts reaction.

4-Chlorophenyl azide is an organic aryl azide compound with the chemical formula C6H4ClN3. The geometry between the nitrogen atoms in the azide functional group is approximately linear while the geometry between the nitrogen and the carbon of the benzene is trigonal planar.

Hydrazobenzene (1,2-diphenylhydrazine) is an aromatic organic compound consisting of two aniline groups joined via their nitrogen atoms. It is an important industrial chemical used in the manufacture of dyes, pharmaceuticals, and hydrogen peroxide.
Ortho effect is an organic chemistry phenomenon where the presence of an chemical group at the at ortho position or the 1 and 2 position of a phenyl ring, relative to the carboxylic compound changes the chemical properties of the compound. This is caused by steric effects and bonding interactions along with polar effects caused by the various substituents which are in a given molecule, resulting in changes in its chemical and physical properties. The ortho effect is associated with substituted benzene compounds.

4,7-Dichloroquinoline is a two-ring heterocyclic compound used as a chemical intermediate to aminoquinoline antimalarial drugs including amodiaquine, chloroquine and hydroxychloroquine.

2-Amino-5-chlorobenzophenone is a substituted benzophenone that can be used in the synthesis of benzodiazepines.

















