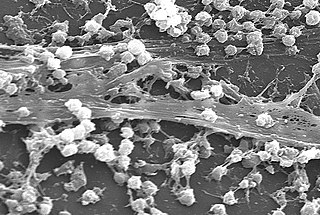
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".
In biology, quorum sensing or quorum signaling (QS) is the ability to detect and respond to cell population density by gene regulation. Quorum sensing is a type of cellular signaling, and more specifically can be considered a type of paracrine signaling. However, it also contains traits of both autocrine signaling: a cell produces both the autoinducer molecule and the receptor for the autoinducer. As one example, QS enables bacteria to restrict the expression of specific genes to the high cell densities at which the resulting phenotypes will be most beneficial, especially for phenotypes that would be ineffective at low cell densities and therefore too energetically costly to express. Many species of bacteria use quorum sensing to coordinate gene expression according to the density of their local population. In a similar fashion, some social insects use quorum sensing to determine where to nest. Quorum sensing in pathogenic bacteria activates host immune signaling and prolongs host survival, by limiting the bacterial intake of nutrients, such as tryptophan, which further is converted to serotonin. As such, quorum sensing allows a commensal interaction between host and pathogenic bacteria. Quorum sensing may also be useful for cancer cell communications.

N-Acyl homoserine lactones are a class of signaling molecules involved in bacterial quorum sensing, a means of communication between bacteria enabling behaviors based on population density.

Pseudomonas aeruginosa is a common encapsulated, Gram-negative, aerobic–facultatively anaerobic, rod-shaped bacterium that can cause disease in plants and animals, including humans. A species of considerable medical importance, P. aeruginosa is a multidrug resistant pathogen recognized for its ubiquity, its intrinsically advanced antibiotic resistance mechanisms, and its association with serious illnesses – hospital-acquired infections such as ventilator-associated pneumonia and various sepsis syndromes.

Stenotrophomonas maltophilia is an aerobic, nonfermentative, Gram-negative bacterium. It is an uncommon bacterium and human infection is difficult to treat. Initially classified as Bacterium bookeri, then renamed Pseudomonas maltophilia, S. maltophilia was also grouped in the genus Xanthomonas before eventually becoming the type species of the genus Stenotrophomonas in 1993.

Filamentation is the anomalous growth of certain bacteria, such as Escherichia coli, in which cells continue to elongate but do not divide. The cells that result from elongation without division have multiple chromosomal copies.

Burkholderia cenocepacia is a Gram-negative, rod-shaped bacterium that is commonly found in soil and water environments and may also be associated with plants and animals, particularly as a human pathogen. It is one of over 20 species in the Burkholderia cepacia complex (Bcc) and is notable due to its virulence factors and inherent antibiotic resistance that render it a prominent opportunistic pathogen responsible for life-threatening, nosocomial infections in immunocompromised patients, such as those with cystic fibrosis or chronic granulomatous disease. The quorum sensing systems CepIR and CciIR regulate the formation of biofilms and the expression of virulence factors such as siderophores and proteases. Burkholderia cenocepacia may also cause disease in plants, such as in onions and bananas. Additionally, some strains serve as plant growth-promoting rhizobacteria.

Xanthomonas campestris is a gram-negative, obligate aerobic bacterium that is a member of the Xanthomonas genus, which is a group of bacteria that are commonly known for their association with plant disease. The species is considered to be dominant amongst its genus, as it originally had over 140 identified pathovars and has been found to infect both monocotyledonous and dicotyledonous plants of economical value with various plant diseases. This includes "black rot" in cruciferous vegetables, bacterial wilt of turfgrass, bacterial blight, and leaf spot, for example.

Stenotrophomonas is a genus of Gram-negative bacteria, comprising at least ten species. The main reservoirs of Stenotrophomonas are soil and plants. Stenotrophomonas species range from common soil organisms to opportunistic human pathogens ; the molecular taxonomy of the genus is still somewhat unclear.

Xanthomonas is a genus of bacteria, many of which cause plant diseases. There are at least 27 plant associated Xanthomonas spp., that all together infect at least 400 plant species. Different species typically have specific host and/or tissue range and colonization strategies.
Autoinducers are signaling molecules that are produced in response to changes in cell-population density. As the density of quorum sensing bacterial cells increases so does the concentration of the autoinducer. Detection of signal molecules by bacteria acts as stimulation which leads to altered gene expression once the minimal threshold is reached. Quorum sensing is a phenomenon that allows both Gram-negative and Gram-positive bacteria to sense one another and to regulate a wide variety of physiological activities. Such activities include symbiosis, virulence, motility, antibiotic production, and biofilm formation. Autoinducers come in a number of different forms depending on the species, but the effect that they have is similar in many cases. Autoinducers allow bacteria to communicate both within and between different species. This communication alters gene expression and allows bacteria to mount coordinated responses to their environments, in a manner that is comparable to behavior and signaling in higher organisms. Not surprisingly, it has been suggested that quorum sensing may have been an important evolutionary milestone that ultimately gave rise to multicellular life forms.
Roberto Kolter is Professor of Microbiology, Emeritus at Harvard Medical School, an author, and past president of the American Society for Microbiology. Kolter has been a professor at Harvard Medical School since 1983 and was Co-director of Harvard's Microbial Sciences Initiative from 2003-2018. During the 35-year term of the Kolter laboratory from 1983 to 2018, more than 130 graduate student and postdoctoral trainees explored an eclectic mix of topics gravitating around the study of microbes. Kolter is a fellow of the American Association for the Advancement of Science and of the American Academy of Microbiology.
Bacterial small RNAs (bsRNA) are small RNAs produced by bacteria; they are 50- to 500-nucleotide non-coding RNA molecules, highly structured and containing several stem-loops. Numerous sRNAs have been identified using both computational analysis and laboratory-based techniques such as Northern blotting, microarrays and RNA-Seq in a number of bacterial species including Escherichia coli, the model pathogen Salmonella, the nitrogen-fixing alphaproteobacterium Sinorhizobium meliloti, marine cyanobacteria, Francisella tularensis, Streptococcus pyogenes, the pathogen Staphylococcus aureus, and the plant pathogen Xanthomonas oryzae pathovar oryzae. Bacterial sRNAs affect how genes are expressed within bacterial cells via interaction with mRNA or protein, and thus can affect a variety of bacterial functions like metabolism, virulence, environmental stress response, and structure.
Interspecies quorum sensing is a type of quorum sensing in which bacteria send and receive signals to other species besides their own. This is accomplished by the secretion of signaling molecules which trigger a response in nearby bacteria at high enough concentrations. Once the molecule hits a certain concentration it triggers the transcription of certain genes such as virulence factors. It has been discovered that bacteria can not only interact via quorum sensing with members of their own species but that there is a kind of universal molecule that allows them to gather information about other species as well. This universal molecule is called autoinducer 2 or AI-2.
Bacterial morphological plasticity refers to changes in the shape and size that bacterial cells undergo when they encounter stressful environments. Although bacteria have evolved complex molecular strategies to maintain their shape, many are able to alter their shape as a survival strategy in response to protist predators, antibiotics, the immune response, and other threats.
Delftia tsuruhatensis is a Gram-negative, rod-shaped, catalase- and oxidase-positive, motile bacterium from the Comamonadaceae family. It was first isolated from a wastewater treatment plant in Japan in 2003. D. tsuruhatensis is an opportunistic and emergent pathogen. All documented human infections are healthcare-associated.
Everett Peter Greenberg is an American microbiologist. He is the inaugural Eugene and Martha Nester Professor of Microbiology at the Department of Microbiology of the University of Washington School of Medicine. He is best known for his research on quorum sensing, and has received multiple awards for his work.

Social motility describes the motile movement of groups of cells that communicate with each other to coordinate movement based on external stimuli. There are multiple varieties of each kingdom that express social motility that provides a unique evolutionary advantages that other species do not possess. This has made them lethal killers such as African trypanosomiasis, or Myxobacteria. These evolutionary advantages have proven to increase survival rate among socially motile bacteria whether it be the ability to evade predators or communication within a swarm to form spores for long term hibernation in times of low nutrients or toxic environments.
Xanthoferrin is an α-hydroxycarboxylate-type of siderophore produced by xanthomonads. Xanthomonas spp. secrete xanthoferrin to chelate iron under low-iron conditions. The xanthoferrin siderophore mediated iron uptake supports bacterial growth under iron-restricted environment.
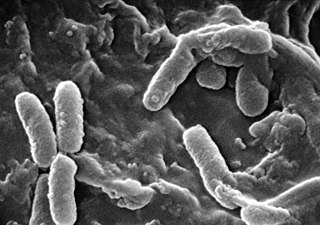
The molecule 2-heptyl-3-hydroxy-4-quinolone, also named the Pseudomonas quinolone signal (PQS), has been discovered as an intracellular link between the two major quorum sensing systems of P. aeruginosa; the las and rhl systems. These systems together control expression of virulence factors and play a major role in the formation of biofilms in Pseudomonas aeruginosa. P. aeruginosa is a gram-negative bacteria and opportunistic human pathogen that can cause serious and sometimes fatal infections in humans. Similar to other bacterial species, P. aeruginosa uses quorum sensing (QS) systems to communicate between cells in a population. This allows coordination of gene expression in a population based on changing cell densities, abundance of nutrients, and other environmental factors.










