
Molecular diffusion often simply called diffusion, is the thermal motion of all particles at temperatures above absolute zero. The rate of this movement is a function of temperature, viscosity of the fluid and the size (mass) of the particles. Diffusion explains the net flux of molecules from a region of higher concentration to one of lower concentration. Once the concentrations are equal the molecules continue to move, but since there is no concentration gradient the process of molecular diffusion has ceased and is instead governed by the process of self-diffusion, originating from the random motion of the molecules. The result of diffusion is a gradual mixing of material such that the distribution of molecules is uniform. Since the molecules are still in motion, but an equilibrium has been established, the result of molecular diffusion is called a "dynamic equilibrium". In a phase with uniform temperature, absent external net forces acting on the particles, the diffusion process will eventually result in complete mixing.

Fick's laws of diffusion describe diffusion and were derived by Adolf Fick in 1855. They can be used to solve for the diffusion coefficient, D. Fick's first law can be used to derive his second law which in turn is identical to the diffusion equation.

Facilitated diffusion is the process of spontaneous passive transport of molecules or ions across a biological membrane via specific transmembrane integral proteins. Being passive, facilitated transport does not directly require chemical energy from ATP hydrolysis in the transport step itself; rather, molecules and ions move down their concentration gradient reflecting its diffusive nature.

Heat transfer is a discipline of thermal engineering that concerns the generation, use, conversion, and exchange of thermal energy (heat) between physical systems. Heat transfer is classified into various mechanisms, such as thermal conduction, thermal convection, thermal radiation, and transfer of energy by phase changes. Engineers also consider the transfer of mass of differing chemical species, either cold or hot, to achieve heat transfer. While these mechanisms have distinct characteristics, they often occur simultaneously in the same system.
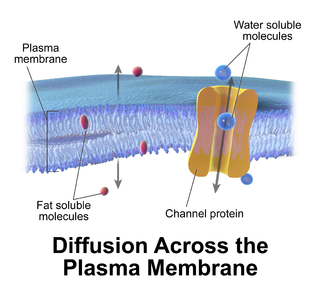
Passive transport is a type of membrane transport that does not require energy to move substances across cell membranes. Instead of using cellular energy, like active transport, passive transport relies on the second law of thermodynamics to drive the movement of substances across cell membranes. Fundamentally, substances follow Fick's first law, and move from an area of high concentration to one of low concentration because this movement increases the entropy of the overall system. The rate of passive transport depends on the permeability of the cell membrane, which, in turn, depends on the organization and characteristics of the membrane lipids and proteins. The four main kinds of passive transport are simple diffusion, facilitated diffusion, filtration, and/or osmosis. Passive transport follow Fick's first law and Second law of thermodynamics
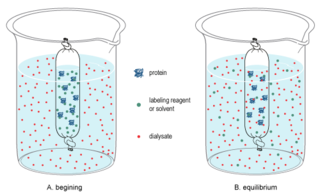
In biochemistry, dialysis is the process of separating molecules in solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing.
In cellular biology, membrane transport refers to the collection of mechanisms that regulate the passage of solutes such as ions and small molecules through biological membranes, which are lipid bilayers that contain proteins embedded in them. The regulation of passage through the membrane is due to selective membrane permeability - a characteristic of biological membranes which allows them to separate substances of distinct chemical nature. In other words, they can be permeable to certain substances but not to others.
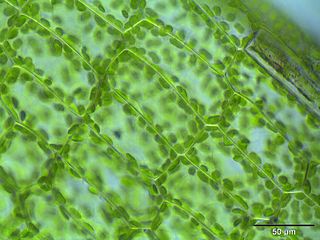
Cytoplasmic streaming, also called protoplasmic streaming and cyclosis, is the flow of the cytoplasm inside the cell, driven by forces from the cytoskeleton. It is likely that its function is, at least in part, to speed up the transport of molecules and organelles around the cell. It is usually observed in large plant and animal cells, greater than approximately 0.1 mm. In smaller cells, the diffusion of molecules is more rapid, but diffusion slows as the size of the cell increases, so larger cells may need cytoplasmic streaming for efficient function.

In chemistry, volatility is a material quality which describes how readily a substance vapourizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast a group of substances evaporate when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate such as dry ice or iodine can vaporize at a similar rate as some liquids under standard conditions.
Mass flow, also known as “mass transfer” and “bulk flow”, is the movement of fluids down a pressure or temperature gradient, particularly in the life sciences. As such, mass flow is a subject of study in both fluid dynamics and biology. Examples of mass flow include blood circulation and transport of water in vascular plant tissues. Mass flow is not to be confused with diffusion which depends on concentration gradients within a medium rather than pressure gradients of the medium itself.

Wood drying reduces the moisture content of wood before its use. When the drying is done in a kiln, the product is known as kiln-dried timber or lumber, whereas air drying is the more traditional method.
In physics and engineering, permeation is the penetration of a permeate through a solid. It is directly related to the concentration gradient of the permeate, a material's intrinsic permeability, and the materials' mass diffusivity. Permeation is modeled by equations such as Fick's laws of diffusion, and can be measured using tools such as a minipermeameter.
The Marangoni number (Ma) is, as usually defined, the dimensionless number that compares the rate of transport due to Marangoni flows, with the rate of transport of diffusion. The Marangoni effect is flow of a liquid due to gradients in the surface tension of the liquid. Diffusion is of whatever is creating the gradient in the surface tension. Thus as the Marangoni number compares flow and diffusion timescales it is a type of Péclet number.

Convection is the transfer of heat from one place to another due to the movement of fluid. Although often discussed as a distinct method of heat transfer, convective heat transfer involves the combined processes of conduction and advection. Convection is usually the dominant form of heat transfer in liquids and gases.

The French flag model is a conceptual definition of a morphogen, described by Lewis Wolpert in the 1960s. A morphogen is defined as a signaling molecule that acts directly on cells to produce specific cellular responses dependent on morphogen concentration. During early development, morphogen gradients generate different cell types in distinct spatial order. French flag patterning is often found in combination with others: vertebrate limb development is one of the many phenotypes exhibiting Turing overlapped with a complementary pattern.
The convection–diffusion equation is a combination of the diffusion and convection (advection) equations, and describes physical phenomena where particles, energy, or other physical quantities are transferred inside a physical system due to two processes: diffusion and convection. Depending on context, the same equation can be called the advection–diffusion equation, drift–diffusion equation, or (generic) scalar transport equation.

Diffusion is the net movement of anything from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in concentration.
In engineering, physics and chemistry, the study of transport phenomena concerns the exchange of mass, energy, charge, momentum and angular momentum between observed and studied systems. While it draws from fields as diverse as continuum mechanics and thermodynamics, it places a heavy emphasis on the commonalities between the topics covered. Mass, momentum, and heat transport all share a very similar mathematical framework, and the parallels between them are exploited in the study of transport phenomena to draw deep mathematical connections that often provide very useful tools in the analysis of one field that are directly derived from the others.
Microvasculature is defined as the microvessels – venules and capillaries of the microcirculation, with a maximum average diameter of 0.3 millimeters. As the vessels decrease in size, they increase their surface-area-to-volume ratio. This allows surface properties to play a significant role in the function of the vessel.
Equimolar counterdiffusion is an instance of molecular diffusion in a binary mixture, and occurs when equal numbers of molecules of the two substances are moving in opposite directions.