Dipole moment may refer to:

In electromagnetism, there are two kinds of dipoles:

Magnetism is a class of physical attributes that are mediated by magnetic fields. Electric currents and the magnetic moments of elementary particles give rise to a magnetic field, which acts on other currents and magnetic moments. Magnetism is one aspect of the combined phenomenon of electromagnetism. The most familiar effects occur in ferromagnetic materials, which are strongly attracted by magnetic fields and can be magnetized to become permanent magnets, producing magnetic fields themselves. Demagnetizing a magnet is also possible. Only a few substances are ferromagnetic; the most common ones are iron, cobalt and nickel and their alloys. The rare-earth metals neodymium and samarium are less common examples. The prefix ferro- refers to iron, because permanent magnetism was first observed in lodestone, a form of natural iron ore called magnetite, Fe3O4.
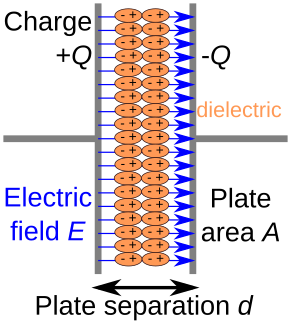
In electromagnetism, a dielectric is an electrical insulator that can be polarized by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor, but instead only slightly shift from their average equilibrium positions, causing dielectric polarization. Because of dielectric polarization, positive charges are displaced in the direction of the field and negative charges shift in the direction opposite to the field. This creates an internal electric field that reduces the overall field within the dielectric itself. If a dielectric is composed of weakly bonded molecules, those molecules not only become polarized, but also reorient so that their symmetry axes align to the field.

The triboelectric effect is a type of contact electrification on which certain materials become electrically charged after they are separated from a different material with which they were in contact. Rubbing the two materials with each other increases the contact between their surfaces, and hence the triboelectric effect. Rubbing glass with fur for example, or a plastic comb through the hair, can build up triboelectricity. Most everyday static electricity is triboelectric. The polarity and strength of the charges produced differ according to the materials, surface roughness, temperature, strain, and other properties.
In physics, a moment is an expression involving the product of a distance and physical quantity, and in this way it accounts for how the physical quantity is located or arranged.

In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
An electret is a dielectric material that has a quasi-permanent electric charge or dipole polarisation. An electret generates internal and external electric fields, and is the electrostatic equivalent of a permanent magnet. Although Oliver Heaviside coined this term in 1885, materials with electret properties were already known to science and had been studied since the early 1700s. One particular example is the electrophorus, a device consisting of a slab with electret properties and a separate metal plate. The electrophorus was originally invented by Johan Carl Wilcke in Sweden and again by Alessandro Volta in Italy.

In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to interaction between the nucleus and electron clouds.

The magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include: loops of electric current, permanent magnets, elementary particles, various molecules, and many astronomical objects.
Nuclear quadrupole resonance spectroscopy or NQR is a chemical analysis technique related to nuclear magnetic resonance (NMR). Unlike NMR, NQR transitions of nuclei can be detected in the absence of a magnetic field, and for this reason NQR spectroscopy is referred to as "zero Field NMR". The NQR resonance is mediated by the interaction of the electric field gradient (EFG) with the quadrupole moment of the nuclear charge distribution. Unlike NMR, NQR is applicable only to solids and not liquids, because in liquids the quadrupole moment averages out. Because the EFG at the location of a nucleus in a given substance is determined primarily by the valence electrons involved in the particular bond with other nearby nuclei, the NQR frequency at which transitions occur is unique for a given substance. A particular NQR frequency in a compound or crystal is proportional to the product of the nuclear quadrupole moment, a property of the nucleus, and the EFG in the neighborhood of the nucleus. It is this product which is termed the nuclear quadrupole coupling constant for a given isotope in a material and can be found in tables of known NQR transitions. In NMR, an analogous but not identical phenomenon is the coupling constant, which is also the result of an internuclear interaction between nuclei in the analyte.
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, inasmuch as matter is made up of elementary particles which have an electric charge, namely protons and electrons. When subject to an electric field, the negatively charged electrons and positively charged atomic nuclei are subject to opposite forces and undergo charge separation. Polarizability is responsible for a material's dielectric constant and, at high (optical) frequencies, its refractive index.
In atomic physics, the electron magnetic moment, or more specifically the electron magnetic dipole moment, is the magnetic moment of an electron caused by its intrinsic properties of spin and electric charge. The value of the electron magnetic moment is approximately −9.284764×10−24 J/T. The electron magnetic moment has been measured to an accuracy of 7.6 parts in 1013.
High resolution electron energy loss spectroscopy (HREELS) is a tool used in surface science. The inelastic scattering of electrons from surfaces is utilized to study electronic excitations or vibrational modes of the surface of a material or of molecules adsorbed to a surface. In contrast to other electron energy loss spectroscopies (EELS), HREELS deals with small energy losses in the range of 10−3 eV to 1 eV. It plays an important role in the investigation of surface structure, catalysis, dispersion of surface phonons and the monitoring of epitaxial growth.

Cohesion or cohesive attraction or cohesive force is the action or property of like molecules sticking together, being mutually attractive. It is an intrinsic property of a substance that is caused by the shape and structure of its molecules, which makes the distribution of surrounding electrons irregular when molecules get close to one another, creating electrical attraction that can maintain a microscopic structure such as a water drop. In other words, cohesion allows for surface tension, creating a "solid-like" state upon which light-weight or low-density materials can be placed.

The bond dipole moment uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges.

In electromagnetism, charge density is the amount of electric charge per unit length, surface area, or volume. Volume charge density is the quantity of charge per unit volume, measured in the SI system in coulombs per cubic meter (C⋅m−3), at any point in a volume. Surface charge density (σ) is the quantity of charge per unit area, measured in coulombs per square meter (C⋅m−2), at any point on a surface charge distribution on a two dimensional surface. Linear charge density (λ) is the quantity of charge per unit length, measured in coulombs per meter (C⋅m−1), at any point on a line charge distribution. Charge density can be either positive or negative, since electric charge can be either positive or negative.
The toroidal ring model, known originally as the Parson magneton or magnetic electron, is a physical model of subatomic particles. It is also known as the plasmoid ring, vortex ring, or helicon ring. This physical model treated electrons and protons as elementary particles, and was first proposed by Alfred Lauck Parson in 1915.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
Magnets exert forces and torques on each other due to the rules of electromagnetism. The forces of attraction field of magnets are due to microscopic currents of electrically charged electrons orbiting nuclei and the intrinsic magnetism of fundamental particles that make up the material. Both of these are modeled quite well as tiny loops of current called magnetic dipoles that produce their own magnetic field and are affected by external magnetic fields. The most elementary force between magnets is the magnetic dipole–dipole interaction. If all of the magnetic dipoles that make up two magnets are known then the net force on both magnets can be determined by summing up all these interactions between the dipoles of the first magnet and that of the second.

The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system, that is, a measure of the system's overall polarity. The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The debye (D) is another unit of measurement used in atomic physics and chemistry.