Related Research Articles

Natural killer cells,also known as NK cells or large granular lymphocytes (LGL),are a type of cytotoxic lymphocyte critical to the innate immune system. They belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans. The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cells,stressed cells,tumor cells,and other intracellular pathogens based on signals from several activating and inhibitory receptors. Most immune cells detect the antigen presented on major histocompatibility complex I (MHC-I) on infected cell surfaces,but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC,allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I. This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells,such as T lymphocyte cells.
A cancer vaccine,or oncovaccine,is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous",being prepared from samples taken from the patient,and are specific to that patient.

Glioblastoma,previously known as glioblastoma multiforme (GBM),is the most aggressive and most common type of cancer that originates in the brain,and has a very poor prognosis for survival. Initial signs and symptoms of glioblastoma are nonspecific. They may include headaches,personality changes,nausea,and symptoms similar to those of a stroke. Symptoms often worsen rapidly and may progress to unconsciousness.
In biology,chimeric antigen receptors (CARs)—also known as chimeric immunoreceptors,chimeric T cell receptors or artificial T cell receptors—are receptor proteins that have been engineered to give T cells the new ability to target a specific antigen. The receptors are chimeric in that they combine both antigen-binding and T cell activating functions into a single receptor.

Cancer immunotherapy (immuno-oncotherapy) is the stimulation of the immune system to treat cancer,improving the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology (immuno-oncology) and a growing subspecialty of oncology.
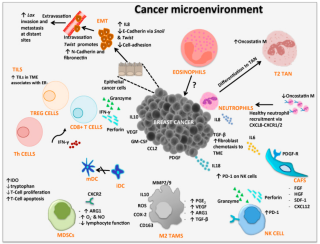
Cancer immunology (immuno-oncology) is an interdisciplinary branch of biology and a sub-discipline of immunology that is concerned with understanding the role of the immune system in the progression and development of cancer;the most well known application is cancer immunotherapy,which utilises the immune system as a treatment for cancer. Cancer immunosurveillance and immunoediting are based on protection against development of tumors in animal systems and (ii) identification of targets for immune recognition of human cancer.
CancerVax was an American pharmaceutical company founded in 1998 by Donald Morton. The company sought to develop a vaccine for cancer,and had candidates for melanoma reach phase III clinical trials. When those trials proved unsuccessful in 2005,the company soon underwent a reverse takeover with Micromet.
Adoptive cell transfer (ACT) is the transfer of cells into a patient. The cells may have originated from the patient or from another individual. The cells are most commonly derived from the immune system with the goal of improving immune functionality and characteristics. In autologous cancer immunotherapy,T cells are extracted from the patient,genetically modified and cultured in vitro and returned to the same patient. Comparatively,allogeneic therapies involve cells isolated and expanded from a donor separate from the patient receiving the cells.
Molecular oncology is an interdisciplinary medical specialty at the interface of medicinal chemistry and oncology that refers to the investigation of the chemistry of cancer and tumors at the molecular scale. Also the development and application of molecularly targeted therapies.

James Patrick Allison is an American immunologist and Nobel laureate who holds the position of professor and chair of immunology and executive director of immunotherapy platform at the MD Anderson Cancer Center in Houston,Texas. Dr. Allison is Regental Professor and Founding-Director of James P. Allison Institute at the MD Anderson Cancer Center.
Prescient Therapeutics Ltd is a clinical stage oncology company. The company is focused on the development of a universal CAR-T platform (OmniCAR),enhanced CAR-T cell manufacturing &function (CellPryme) and on two small molecule drug targeted therapies.

Carl H. June is an American immunologist and oncologist. He is currently the Richard W. Vague Professor in Immunotherapy in the Department of Pathology and Laboratory Medicine at the Perelman School of Medicine of the University of Pennsylvania. He is most well known for his research on T cell therapies for the treatment of several forms of cancers. In 2020 he was elected to the American Philosophical Society.

Crystal L. Mackall is an American physician and immunologist. She is currently the Ernest and Amelia Gallo Family Professor of Pediatrics and Medicine at Stanford University. She is the founding director of the Stanford Center for Cancer Cell Therapy.
Misty Rayna Jenkins is an Australian scientist known for her research into lymphocytes and cancer treatment.
Peter Edward Fecci is an American neurosurgeon,professor and researcher. He is an Associate Professor of Neurosurgery,Pathology and Immunology at Duke University School of Medicine. He also serves as Director of the Duke Center for Brain and Spine Metastasis,Director of the Brain Tumor Immunotherapy Program,Residency Program Director,and Associate Deputy Director of the Preston Robert Tisch Brain Tumor Center at Duke.
Marcela V. Maus is a professor of medicine at Harvard Medical School and director of the Cellular Immunotherapy Program at Massachusetts General Hospital. She works on immunotherapy for the treatment of cancer,using genetically engineered T cells to target malignancies (cancer).

Duane A. Mitchell is an American physician-scientist and university professor. He is currently employed at the University of Florida College of Medicine,in Gainesville,Florida as the Assistant Vice President for Research,Associate Dean for Translational Science and Clinical Research,and Director of the University of Florida (UF) Clinical and Translational Science Institute. He is the Phyllis Kottler Friedman Professor in the Lillian S. Wells Department of Neurosurgery. and co-director of the Preston A. Wells Jr. Center for Brain Tumor Therapy. Mitchell is also the founder,President,and Chairman of iOncologi,Inc.,a biotechnology company in Gainesville,FL specializing in immuno-oncology.

Cellular adoptive immunotherapy is a type of immunotherapy. Immune cells such as T-cells are usually isolated from patients for expansion or engineering purposes and reinfused back into patients to fight diseases using their own immune system. A major application of cellular adoptive therapy is cancer treatment,as the immune system plays a vital role in the development and growth of cancer. The primary types of cellular adoptive immunotherapies are T cell therapies. Other therapies include CAR-T therapy,CAR-NK therapy,macrophage-based immunotherapy and dendritic cell therapy.
Engineered chimeric antigen receptor (CAR)-T cell delivery is the methodology by which clinicians introduce the cancer-targeting therapeutic system of the CAR-T cell to the human body. CAR-T cells,which utilizes genetic modification of human T-cells to contain antigen binding sequences in addition to the receptor systems CD4 or CD8,are useful in direct targeting and elimination of cancer cells through cytotoxicity.
KHYG-1 is an immortalized cell line that bears the characteristics of NK cells. NK cells are a type of immune cell that are found in blood whose innate function is to kill viral infected cells,cells under stress and cancer cells. The KHYG-1 cell line was established in 1997 in the laboratory of M Yagita in the department of Clinical Immunology and Haematology,Tazuke-Kofukai Medical Research Institute,Kitano Hospital,Osaka,Japan. These cells were derived from the blood of 45-year old female suffering from aggressive Natural killer cell lymphoblastic leukemia/lymphoma. This cell line has been growing continuously,in the presence of IL-2,for 18 months after isolation and its doubling time is around 24-48h. The ability to proliferate was retained even after cryopreservation in liquid nitrogen.
References
- ↑ "Combining CAR T cells with existing immunotherapies may overcome resistance in glioblastomas".
- ↑ "Donald M. O'rourke | Faculty | About Us | Perelman School of Medicine | Perelman School of Medicine at the University of Pennsylvania". www.med.upenn.edu. Retrieved 2023-01-12.
- ↑ "Personnel | O'Rourke Lab | Perelman School of Medicine at the University of Pennsylvania".
- ↑ "New Penn Medicine Center Brings Immunotherapy Research to Brain Tumor Treatment".
- ↑ "CAR T-cell therapy 'quite promising' for glioblastoma".
- ↑ "Combining CAR T cells with existing immunotherapies may overcome resistance in glioblastomas".
- ↑ O'Rourke, Donald M., et al. "A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma." Science translational medicine 9.399 (2017): eaaa0984.