Related Research Articles

Antiviral drugs are a class of medication used for treating viral infections. Most antivirals target specific viruses, while a broad-spectrum antiviral is effective against a wide range of viruses. Antiviral drugs are one class of antimicrobials, a larger group which also includes antibiotic, antifungal and antiparasitic drugs, or antiviral drugs based on monoclonal antibodies. Most antivirals are considered relatively harmless to the host, and therefore can be used to treat infections. They should be distinguished from virucides, which are not medication but deactivate or destroy virus particles, either inside or outside the body. Natural virucides are produced by some plants such as eucalyptus and Australian tea trees.

David Da-i Ho is a Taiwanese-American AIDS researcher, physician, and virologist who has made a number of scientific contributions to the understanding and treatment of HIV infection. He championed for combination anti-retroviral therapy instead of single therapy, which turned HIV from absolute terminal disease into a chronic disease.
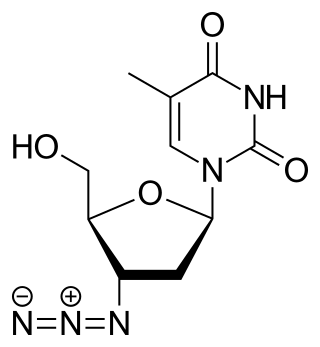
Zidovudine (ZDV), also known as azidothymidine (AZT), is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use in combination with other antiretrovirals. It may be used to prevent mother-to-child spread during birth or after a needlestick injury or other potential exposure. It is sold both by itself and together as lamivudine/zidovudine and abacavir/lamivudine/zidovudine. It can be used by mouth or by slow injection into a vein.
The management of HIV/AIDS normally includes the use of multiple antiretroviral drugs as a strategy to control HIV infection. There are several classes of antiretroviral agents that act on different stages of the HIV life-cycle. The use of multiple drugs that act on different viral targets is known as highly active antiretroviral therapy (HAART). HAART decreases the patient's total burden of HIV, maintains function of the immune system, and prevents opportunistic infections that often lead to death. HAART also prevents the transmission of HIV between serodiscordant same-sex and opposite-sex partners so long as the HIV-positive partner maintains an undetectable viral load.
Reverse-transcriptase inhibitors (RTIs) are a class of antiretroviral drugs used to treat HIV infection or AIDS, and in some cases hepatitis B. RTIs inhibit activity of reverse transcriptase, a viral DNA polymerase that is required for replication of HIV and other retroviruses.
The Division of Acquired Immunodeficiency Syndrome (DAIDS) is a division of the National Institute of Allergy and Infectious Diseases, which is part of the National Institutes of Health. It was formed in 1986 as a part of the initiative to address the national research needs created by the advent and spread of the HIV/AIDS epidemic. Specifically, the Division's mission is to increase basic knowledge of the pathogenesis, natural history, and transmission of HIV disease and to support research that promotes progress in its detection, treatment, and prevention. DAIDS accomplishes this through planning, implementing, managing, and evaluating programs in (1) fundamental basic research, (2) discovery and development of therapies for HIV infection and its complications, and (3) discovery and development of vaccines and other prevention strategies.
HIV superinfection is a condition in which a person with an established human immunodeficiency virus infection acquires a second strain of HIV, often of a different subtype. These can form a recombinant strain that co-exists with the strain from the initial infection, as well from reinfection with a new virus strain, and may cause more rapid disease progression or carry multiple resistances to certain HIV medications.

A resistance mutation is a mutation in a virus gene that allows the virus to become resistant to treatment with a particular antiviral drug. The term was first used in the management of HIV, the first virus in which genome sequencing was routinely used to look for drug resistance. At the time of infection, a virus will infect and begin to replicate within a preliminary cell. As subsequent cells are infected, random mutations will occur in the viral genome. When these mutations begin to accumulate, antiviral methods will kill the wild type strain, but will not be able to kill one or many mutated forms of the original virus. At this point a resistance mutation has occurred because the new strain of virus is now resistant to the antiviral treatment that would have killed the original virus. Resistance mutations are evident and widely studied in HIV due to its high rate of mutation and prevalence in the general population. Resistance mutation is now studied in bacteriology and parasitology.
HIV drug resistance occurs when microevolution causes virions to become tolerant to antiretroviral treatments (ART). ART can be used to successfully manage HIV infection, but a number of factors can contribute to the virus mutating and becoming resistant. Drug resistance occurs as bacterial or viral populations evolve to no longer respond to medications that previously worked. In the case of HIV, there have been recognized cases of treatment resistant strains since 1989, with drug resistance being a major contributor to treatment failure. While global incidence varies greatly from region to region, there has been a general increase in overall HIV drug resistance. The two main types of resistance, primary and induced, differ mostly in causation, with the biggest cause of resistance being a lack of adherence to the specific details of treatment. These newly created resistant strains of HIV pose a public health issue as they infect a growing number of people because they are harder to treat, and can be spread to other individuals. For this reason, the reaction to the growing number of cases of resistant HIV strains has mostly been to try to increase access to treatment and implement other measures to make sure people stay in care, as well as to look into the development of an HIV vaccine or cure.
Joel N. Blankson is a professor at the Johns Hopkins School of Medicine in the Department of Medicine, Division of Infectious Diseases. Blankson is an expert on HIV infection, particularly HIV latency and long-term control of HIV infection. He is a lead investigator in studies on these topics and is frequently interviewed in the scientific and popular press. Blankson also practices internal and infectious diseases medicine in Lutherville, Maryland.
HIV Resistance Response Database Initiative (RDI) is a not-for-profit organisation established in 2002 which states its mission as "To improve the clinical management of HIV infection by developing a large clinical database and bioinformatic techniques that predict accurately any individual's response to any combination of HIV drugs."

HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
Julio S. G. Montaner, is an Argentine-Canadian physician, professor and researcher. He is the director of the British Columbia Centre for Excellence in HIV/AIDS, the chair in AIDS Research and head of the Division of AIDS in the Faculty of Medicine at the University of British Columbia and the past-president of the International AIDS Society. He is also the director of the John Ruedy Immunodeficiency Clinic, and the Physician Program Director for HIV/AIDS PHC. He is known for his work on HAART, a role in the discovery of triple therapy as an effective treatment for HIV in the late 1990s, and a role in advocating the "Treatment as Prevention" Strategy in the mid-2000s, led by Myron Cohen of the HPTN 052 trial.
Deborah Persaud is a Guyanese-born American virologist who primarily works on HIV/AIDS at Johns Hopkins Children's Center.
Viral load monitoring for HIV is the regular measurement of the viral load of individual HIV-positive people as part of their personal plan for treatment of HIV/AIDS. A count of the viral load is routine before the start of HIV treatment.

Sharon Ruth Lewin, FRACP, FAHMS is the inaugural Director of The Peter Doherty Institute for Infection and Immunity. She is also a Professor of Medicine at The University of Melbourne, a National Health and Medical Research Council (NHMRC) Practitioner Fellow, Director of the Cumming Global Centre for Pandemic Therapeutics, and President of the International AIDS Society (IAS).
Virological failure is defined as the failure to meet a specific target of antiviral drug treatment, namely the non-attainment or non-maintenance of undetectable viral load, particularly in the treatment of HIV. As antiretroviral therapy is evaluated by detecting the amount of copies of the virus in blood samples, the concept of virological failure gives a way to modify treatment of this disease.

Diane Havlir is an American physician who is a Professor of Medicine and Chief of the HIV/AIDS Division at the University of California, San Francisco. Her research considers novel therapeutic strategies to improve the lives of people with HIV and to support public health initiatives in East Africa. She was elected to the National Academy of Medicine in 2019.

Mary F. Kearney is an American biologist. She is a senior scientist and head of the translational research section in the HIV dynamics and replication program at the National Cancer Institute.

Charles Williams Flexner is an American physician, clinical pharmaceutical scientist, academic, author and researcher. He is a Professor of Medicine at the Johns Hopkins University School of Medicine.
References
- ↑ Larder, B; Darby, G; Richman, DD (1989). "HIV with reduced sensitivity to zidovudine isolated during prolonged therapy". Science. 243 (4899): 1731–1734. doi:10.1126/science.2467383. PMID 2467383.
- ↑ Wong, JK; Hezareh, M; Gunthard, HF; Havlir, DV; Ignacio, CC; Spina, CA; Richman, DD (1997). "Recovery of replication-competent HIV despite prolonged suppression of plasma viremia" (PDF). Science. 278 (5341): 1291–95. doi:10.1126/science.278.5341.1291. PMID 9360926.
- ↑ Richman, DD; Wrin, T; Little, SJ; Petropoulos, CJ (2003). "Rapid evolution of the neutralizing antibody response to HIV type 1 infection". Proc Natl Acad Sci U S A. 100 (7): 4144–9. Bibcode:2003PNAS..100.4144R. doi: 10.1073/pnas.0630530100 . PMC 153062 . PMID 12644702.
- ↑ Richman DD, Whitley RJ, Hayden FG. Clinical Virology, Edited by Richman DD, Whitley RJ, Hayden FG. 4th Edition. ASM Press Washington DC; 1-1489, 2017.
- ↑ Anon (2003). "Dr. Douglas Richman wins VA's Middleton Award". J Investig Med. 51 (3): 127. doi:10.1136/jim-51-03-14. PMID 12769194. S2CID 29299691.
- ↑ Vere Hodge, R. Anthony, ed. (2019). The International Society for Antiviral Research: The Third Decade 2008-2017 (PDF). International Society for Antiviral Research.
- ↑ Bouchrika, Imed. "douglas-d-richman". Research.com. Retrieved 26 June 2023.