Related Research Articles
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent called the mobile phase, which carries it through a system on which a material called the stationary phase is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify specific components in mixtures. The mixtures can originate from food, chemicals, pharmaceuticals, biological, environmental and agriculture, etc, which have been dissolved into liquid solutions.

Ion chromatography is a form of chromatography that separates ions and ionizable polar molecules based on their affinity to the ion exchanger. It works on almost any kind of charged molecule—including small inorganic anions, large proteins, small nucleotides, and amino acids. However, ion chromatography must be done in conditions that are one pH unit away from the isoelectric point of a protein.

Thin-layer chromatography (TLC) is a chromatography technique that separates components in non-volatile mixtures.

Liquid chromatography–mass spectrometry (LC–MS) is an analytical chemistry technique that combines the physical separation capabilities of liquid chromatography with the mass analysis capabilities of mass spectrometry (MS). Coupled chromatography – MS systems are popular in chemical analysis because the individual capabilities of each technique are enhanced synergistically. While liquid chromatography separates mixtures with multiple components, mass spectrometry provides spectral information that may help to identify each separated component. MS is not only sensitive, but provides selective detection, relieving the need for complete chromatographic separation. LC–MS is also appropriate for metabolomics because of its good coverage of a wide range of chemicals. This tandem technique can be used to analyze biochemical, organic, and inorganic compounds commonly found in complex samples of environmental and biological origin. Therefore, LC–MS may be applied in a wide range of sectors including biotechnology, environment monitoring, food processing, and pharmaceutical, agrochemical, and cosmetic industries. Since the early 2000s, LC–MS has also begun to be used in clinical applications.

Solid-phase extraction (SPE) is a solid-liquid extractive technique, by which compounds that are dissolved or suspended in a liquid mixture are separated, isolated or purified, from other compounds in this mixture, according to their physical and chemical properties. Analytical laboratories use solid phase extraction to concentrate and purify samples for analysis. Solid phase extraction can be used to isolate analytes of interest from a wide variety of matrices, including urine, blood, water, beverages, soil, and animal tissue.
Chiral column chromatography is a variant of column chromatography that is employed for the separation of chiral compounds, i.e. enantiomers, in mixtures such as racemates or related compounds. The chiral stationary phase (CSP) is made of a support, usually silica based, on which a chiral reagent or a macromolecule with numerous chiral centers is bonded or immobilized.
Countercurrent distribution is an analytical chemistry technique which was developed by Lyman C. Craig in the 1940s. Countercurrent distribution is a separation process that is founded on the principles of liquid–liquid extraction where a chemical compound is distributed (partitioned) between two immiscible liquid phases according to its relative solubility in the two phases. The simplest form of liquid-liquid extraction is the partitioning of a mixture of compounds between two immiscible liquid phases in a separatory funnel. This occurs in five steps: 1) preparation of the separatory funnel with the two phase solvent system, 2) introduction of the compound mixture into the separatory funnel, 3) vigorous shaking of the separatory funnel to mix the two layers and allow for mass transfer of compounds in and out of the phases, 4) The contents of the separatory funnel are allowed to settle back into two distinct phases and 5) the two phases are separated from each other by draining out the bottom phase. If a compound is insoluble in the lower phase it will distribute into the upper phase and stay in the separatory funnel. If a compound is insoluble in the upper phase it will distribute into the lower phase and be removed from the separatory funnel. If the mixture contains one or more compounds that are soluble in the upper phase and one or more compounds that are soluble in the lower phase, then an extraction has occurred. Often, an individual compound is soluble to a certain extent in both phases and the extraction is, therefore, incomplete. The relative solubility of a compound in two phases is known as the partition coefficient.
Reversed-phase Liquid chromatography (RP-LC) is a mode of liquid chromatography in which non-polar stationary phase and polar mobile phases are used for the separation of organic compounds. The vast majority of separations and analyses using High Performance Liquid Chromatography-HPLC in recent years are done using the Reversed Phase mode. In the Reversed Phase mode, the sample components are retained in the system, the more hydrophobic they are.
Mixed-mode chromatography (MMC), or multimodal chromatography, refers to chromatographic methods that utilize more than one form of interaction between the stationary phase and analytes in order to achieve their separation. What is distinct from conventional single-mode chromatography is that the secondary interactions in MMC cannot be too weak, and thus they also contribute to the retention of the solutes.
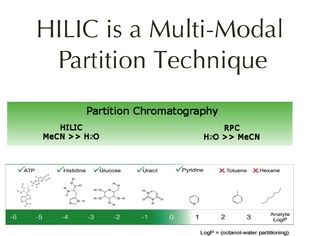
Hydrophilic interaction chromatography is a variant of normal phase liquid chromatography that partly overlaps with other chromatographic applications such as ion chromatography and reversed phase liquid chromatography. HILIC uses hydrophilic stationary phases with reversed-phase type eluents. The name was suggested by Andrew Alpert in his 1990 paper on the subject. He described the chromatographic mechanism for it as liquid-liquid partition chromatography where analytes elute in order of increasing polarity, a conclusion supported by a review and re-evaluation of published data.
Supercritical fluid chromatography (SFC) is a form of normal phase chromatography that uses a supercritical fluid such as carbon dioxide as the mobile phase. It is used for the analysis and purification of low to moderate molecular weight, thermally labile molecules and can also be used for the separation of chiral compounds. Principles are similar to those of high performance liquid chromatography (HPLC), however SFC typically utilizes carbon dioxide as the mobile phase; therefore the entire chromatographic flow path must be pressurized. Because the supercritical phase represents a state whereby bulk liquid and gas properties converge, supercritical fluid chromatography is sometimes called convergence chromatography. The idea of liquid and gas properties convergence was first envisioned by Giddings.
Micellar liquid chromatography (MLC) is a form of reversed phase liquid chromatography that uses an aqueous micellar solutions as the mobile phase.
Electrochromatography is a chemical separation technique in analytical chemistry, biochemistry and molecular biology used to resolve and separate mostly large biomolecules such as proteins. It is a combination of size exclusion chromatography and gel electrophoresis. These separation mechanisms operate essentially in superposition along the length of a gel filtration column to which an axial electric field gradient has been added. The molecules are separated by size due to the gel filtration mechanism and by electrophoretic mobility due to the gel electrophoresis mechanism. Additionally there are secondary chromatographic solute retention mechanisms.
Sample preparation for mass spectrometry is used for the optimization of a sample for analysis in a mass spectrometer (MS). Each ionization method has certain factors that must be considered for that method to be successful, such as volume, concentration, sample phase, and composition of the analyte solution. Quite possibly the most important consideration in sample preparation is knowing what phase the sample must be in for analysis to be successful. In some cases the analyte itself must be purified before entering the ion source. In other situations, the matrix, or everything in the solution surrounding the analyte, is the most important factor to consider and adjust. Often, sample preparation itself for mass spectrometry can be avoided by coupling mass spectrometry to a chromatography method, or some other form of separation before entering the mass spectrometer. In some cases, the analyte itself must be adjusted so that analysis is possible, such as in protein mass spectrometry, where usually the protein of interest is cleaved into peptides before analysis, either by in-gel digestion or by proteolysis in solution.
Partition chromatography theory and practice was introduced through the work and publications of Archer Martin and Richard Laurence Millington Synge during the 1940s. They would later receive the 1952 Nobel Prize in Chemistry "for their invention of partition chromatography".

Bobgunnia madagascariensis, also called the snake bean plant, is a species of legume in the family Fabaceae.

Countercurrent chromatography is a form of liquid–liquid chromatography that uses a liquid stationary phase that is held in place by inertia of the molecules composing the stationary phase accelerating toward the center of a centrifuge due to centripetal force and is used to separate, identify, and quantify the chemical components of a mixture. In its broadest sense, countercurrent chromatography encompasses a collection of related liquid chromatography techniques that employ two immiscible liquid phases without a solid support. The two liquid phases come in contact with each other as at least one phase is pumped through a column, a hollow tube or a series of chambers connected with channels, which contains both phases. The resulting dynamic mixing and settling action allows the components to be separated by their respective solubilities in the two phases. A wide variety of two-phase solvent systems consisting of at least two immiscible liquids may be employed to provide the proper selectivity for the desired separation.
Centrifugal partition chromatography is a special chromatographic technique where both stationary and mobile phase are liquid, and the stationary phase is immobilized by a strong centrifugal force. Centrifugal partition chromatography consists of a series-connected network of extraction cells, which operates as elemental extractors, and the efficiency is guaranteed by the cascade.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.
References
- 1 2 Tanimura, T.; Pisano, J. J.; Ito, Y.; Bowman, R. L. (1970). "Droplet Countercurrent Chromatography". Science. 169 (3940): 54–56. Bibcode:1970Sci...169...54T. doi:10.1126/science.169.3940.54. PMID 5447530. S2CID 32380725.
- 1 2 Hostettmann, Kurt (1980). "Droplet Counter-Current Chromatography and its Application to the Preparative Scale Separation of Natural Products". Planta Medica. 39 (5): 1–18. doi: 10.1055/s-2008-1074898 .
- ↑ Friesen, J. Brent; McAlpine, James B.; Chen, Shao-Nong; Pauli, Guido F. (2015). "Countercurrent Separation of Natural Products: An Update". Journal of Natural Products. 78 (7): 1765–1796. doi:10.1021/np501065h. PMC 4517501 . PMID 26177360.
- 1 2 3 Ogihara, Yukio; Inoue, Osamu; Otsuka, Hideaki; Kawai, Ken-Ichi; Tanimura, Takenori; Shibata, Shoji (1976). "Droplet counter-current chromatography for the separation of plant products". Journal of Chromatography A. 128 (1): 218–223. doi:10.1016/S0021-9673(00)84058-3.
- 1 2 Francis, G. W.; Isaksen, M. (1989). "Droplet counter current chromatography of the carotenoids of parsley Petroselinum crispum". Chromatographia. 27 (11–12): 549–551. doi:10.1007/BF02258976. S2CID 59391286.
- ↑ Domon, Bruno; Hostettmann, Maryse; Hostettmann, Kurt (1982). "Droplet counter-current chromatography with non-aqueous solvent systems". Journal of Chromatography A. 246 (1): 133–135. doi:10.1016/S0021-9673(00)82791-0.
- ↑ Kies, Marian W.; Davis, Michael G. (1951). "A New Procedure for Fractionation of Mixtures by Solvent Distribution" (PDF). Journal of Biological Chemistry. 189 (2): 637–650. doi: 10.1016/S0021-9258(18)44880-6 . PMID 14832281 . Retrieved 2016-02-27.
- 1 2 Conway, Walter D. (1990). "The Evolution of Countercurrent Chromatography". Countercurrent Chromatography: Apparatus,Theory, & Applications. VCH. pp. 37–115. ISBN 978-0-89573-331-3.
- ↑ Kostanyan, A. E.; Voshkin, A. A.; Kodin, N. V. (2011). "Pulsed cyclic device for liquid countercurrent chromatography". Theoretical Foundations of Chemical Engineering. 45 (5): 779–785. doi:10.1134/S0040579511050095. S2CID 98467011.
- ↑ Kostanyan, Artak E.; Voshkin, Andrei A.; Kodin, Nikolai V. (2011). "Controlled-cycle pulsed liquid–liquid chromatography. A modified version of Craig's counter-current distribution". Journal of Chromatography A. 1218 (36): 6135–6143. doi:10.1016/j.chroma.2010.12.103. PMID 21281934.
- ↑ Hostettmann, Kurt; Hostettmann-Kaldas, Maryse; Sticher, Otto (1979). "Application of droplet counter-current chromatography to the isolation of natural products". Journal of Chromatography A. 186: 529–534. doi:10.1016/S0021-9673(00)95273-7.
- ↑ Hostettmann, K.; Appolonia, C.; Domon, B.; Hostettmann, M. (1984). "Droplet Countercurrent Chromatography - New Applications in Natural Products Chemistry". Journal of Liquid Chromatography. 7 (2): 231–242. doi:10.1080/01483918408073964.
- ↑ Hosteeman, Kurt; Marston, Andrew (1988). "Natural Products Isolation of Droplet Countercurrent Chromatography". Countercurrent Chromatography: Theory and Practice. Chromatographic science series. Vol. 44. Marcel Dekker. pp. 465–492. ISBN 978-0-8247-7815-6.
- ↑ Kawai, Ken-Ichi; Akiyama, Toshiyuki; Ogihara, Yukio; Shibata, Shoji (1974). "A new sapogenin in the saponins of Zizyphus jujuba, Hovenia dulcis and Bacopa monniera". Phytochemistry. 13 (12): 2829–2832. Bibcode:1974PChem..13.2829K. doi:10.1016/0031-9422(74)80250-5.
- ↑ Otsuka, Hideaki; Ogihara, Yukio; Shibata, Shoji (1974). "Isolation of coclaurine from Zizyphus jujuba by droplet counter-current chromatography". Phytochemistry. 13 (9): 2016. Bibcode:1974PChem..13.2016O. doi:10.1016/0031-9422(74)85153-8.
- 1 2 Hostettmann, Kurt; Hostettmann-Kaldas, Maryse; Nakanishi, Koji (1979). "Droplet counter-current chromatography for the preparative isolation of various glycosides". Journal of Chromatography A. 170 (2): 355–361. doi:10.1016/S0021-9673(00)95460-8.
- 1 2 Hostettmann, Kurt; Hostettmann-Kaldas, Maryse; Sticher, Otto (1979). "Preparative Scale Separation of Xanthones and Iridoid Glycosides by Droplet Counter-Current Chromatography". Helvetica Chimica Acta. 62 (7): 2079–2085. doi:10.1002/hlca.19790620705.
- ↑ Kurumaya, Katsuyuki; Sakamoto, Tetsuto; Okada, Yoshihito; Kajiwara, Masahiro (1988). "Application of droplet counter-current chromatography to the isolation of vitamin B12". Journal of Chromatography A. 435 (1): 235–240. doi:10.1016/S0021-9673(01)82181-6. PMID 3350896.
- ↑ Sousa, Adriana L.; Sales, Queitilane S.; Braz-Filho, Raimundo; de Oliveira, Rodrigo R. (2012). "Lignans and Flavonoids isolated from Cuscuta racemosa MART. & HUMB (Convolulaceae) by droplet counter-current chromatography". Journal of Liquid Chromatography & Related Technologies. 35 (16): 2294–2303. doi:10.1080/10826076.2011.631259. S2CID 94294767.
- ↑ De Marino, Simona; Cattaneo, Fabio; Festa, Carmen; Zollo, Franco; Iaccio, Annalisa; Ammendola, Rosario; Incollingo, Filomena; Iorizzi, Maria (2011). "Imbricatolic Acid from Juniperus communis L. Prevents Cell Cycle Progression in CaLu-6 Cells". Planta Medica. 77 (16): 1822–1828. doi:10.1055/s-0030-1271104. PMID 21567359. S2CID 260251906.
- ↑ Dini, Irene (2011). "Flavonoid glycosides from Pouteria obovata (R. Br.) fruit flour". Food Chemistry. 124 (3): 884–888. doi:10.1016/j.foodchem.2010.07.013.
- ↑ Nasser, A. L. M.; Mazzolin, L. P.; Hiruma-Lima, C. A.; Santos, L. S.; Eberlin, M. N.; Souza Brito, A. R. Monteiro; Vilegas, W. (2006). "Preparative Droplet Counter-Current Chromatography for the Separation of the New Nor-Seco-Triterpene and Pentacyclic Triterpenoids from Qualea Parviflora". Chromatographia. 64 (11–12): 695–699. doi:10.1365/s10337-006-0087-4. S2CID 96557342.