
A non-coding RNA (ncRNA) is a functional RNA molecule that is not translated into a protein. The DNA sequence from which a functional non-coding RNA is transcribed is often called an RNA gene. Abundant and functionally important types of non-coding RNAs include transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs), as well as small RNAs such as microRNAs, siRNAs, piRNAs, snoRNAs, snRNAs, exRNAs, scaRNAs and the long ncRNAs such as Xist and HOTAIR.
The 5′ untranslated region is the region of a messenger RNA (mRNA) that is directly upstream from the initiation codon. This region is important for the regulation of translation of a transcript by differing mechanisms in viruses, prokaryotes and eukaryotes. While called untranslated, the 5′ UTR or a portion of it is sometimes translated into a protein product. This product can then regulate the translation of the main coding sequence of the mRNA. In many organisms, however, the 5′ UTR is completely untranslated, instead forming a complex secondary structure to regulate translation.

Dosage compensation is the process by which organisms equalize the expression of genes between members of different biological sexes. Across species, different sexes are often characterized by different types and numbers of sex chromosomes. In order to neutralize the large difference in gene dosage produced by differing numbers of sex chromosomes among the sexes, various evolutionary branches have acquired various methods to equalize gene expression among the sexes. Because sex chromosomes contain different numbers of genes, different species of organisms have developed different mechanisms to cope with this inequality. Replicating the actual gene is impossible; thus organisms instead equalize the expression from each gene. For example, in humans, female (XX) cells randomly silence the transcription of one X chromosome, and transcribe all information from the other, expressed X chromosome. Thus, human females have the same number of expressed X-linked genes per cell as do human males (XY), both sexes having essentially one X chromosome per cell, from which to transcribe and express genes.

X-inactivation is a process by which one of the copies of the X chromosome is inactivated in therian female mammals. The inactive X chromosome is silenced by being packaged into a transcriptionally inactive structure called heterochromatin. As nearly all female mammals have two X chromosomes, X-inactivation prevents them from having twice as many X chromosome gene products as males, who only possess a single copy of the X chromosome.
P elements are transposable elements that were discovered in Drosophila as the causative agents of genetic traits called hybrid dysgenesis. The transposon is responsible for the P trait of the P element and it is found only in wild flies. They are also found in many other eukaryotes.

X hyperactivation refers to the process in Drosophila by which genes on the X chromosome in male flies become twice as active as genes on the X chromosome in female flies.

The ZW sex-determination system is a chromosomal system that determines the sex of offspring in birds, some fish and crustaceans such as the giant river prawn, some insects, the schistosome family of flatworms, and some reptiles, e.g. majority of snakes, lacertid lizards and monitors, including Komodo dragons. It is also present in some plants, where it has probably evolved independently on several occasions. The letters Z and W are used to distinguish this system from the XY sex-determination system. In the ZW system, females have a pair of dissimilar ZW chromosomes, and males have two similar ZZ chromosomes.
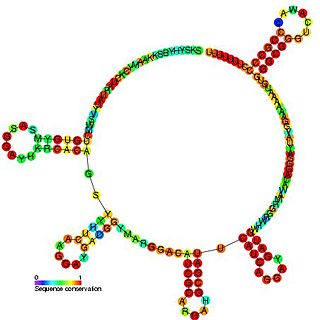
The PrrB/RsmZ RNA family are a group of related non-coding RNA molecules found in bacteria. PrrB RNA is able to phenotypically complement gacS and gacA mutants and is itself regulated by the GacS-GacA two-component signal transduction system. Inactivation of the prrB gene in Pseudomonas fluorescens F113 resulted in a significant reduction of 2, 4-diacetylphloroglucinol (Phl) and hydrogen cyanide (HCN) production, while increased metabolite production was observed when prrB was overexpressed. The prrB gene sequence contains a number of imperfect repeats of the consensus sequence 5′-AGGA-3′, and sequence analysis predicted a complex secondary structure featuring multiple putative stem-loops with the consensus sequences predominantly positioned at the single-stranded regions at the ends of the stem-loops. This structure is similar to the CsrB and RsmB regulatory RNAs, suggesting this RNA also interacts with a CsrA-like protein.
The gene extramachrochaetae (emc) is a Drosophila melanogaster gene that codes for the Emc protein, which has a wide variety of developmental roles. It was named, as is common for Drosophila genes, after the phenotypic change caused by a mutation in the gene (macrochaetae are the longer bristles on Drosophila).

Histone RNA hairpin-binding protein or stem-loop binding protein (SLBP) is a protein that in humans is encoded by the SLBP gene.

Xist is a non-coding RNA transcribed from the X chromosome of the placental mammals that acts as a major effector of the X-inactivation process. It is a component of the Xic – X-chromosome inactivation centre – along with two other RNA genes and two protein genes.

Long non-coding RNAs are a type of RNA, generally defined as transcripts more than 200 nucleotides that are not translated into protein. This arbitrary limit distinguishes long ncRNAs from small non-coding RNAs, such as microRNAs (miRNAs), small interfering RNAs (siRNAs), Piwi-interacting RNAs (piRNAs), small nucleolar RNAs (snoRNAs), and other short RNAs. Given that some lncRNAs have been reported to have the potential to encode small proteins or micro-peptides, the latest definition of lncRNA is a class of RNA molecules of over 200 nucleotides that have no or limited coding capacity. Long intervening/intergenic noncoding RNAs (lincRNAs) are sequences of lncRNA which do not overlap protein-coding genes.

Tsix is a non-coding RNA gene that is antisense to the Xist RNA. Tsix binds Xist during X chromosome inactivation. The name Tsix comes from the reverse of Xist, which stands for X-inactive specific transcript.
Gene gating is a phenomenon by which transcriptionally active genes are brought next to nuclear pore complexes (NPCs) so that nascent transcripts can quickly form mature mRNA associated with export factors. Gene gating was first hypothesised by Günter Blobel in 1985. It has been shown to occur in Saccharomyces cerevisiae, Caenorhabditis elegans, Drosophila melanogaster as well as mammalian model systems.
Epigenetics of human development is the study of how epigenetics effects human development.
Mitzi Kuroda is a professor of genetics at Harvard Medical School and Brigham and Women's Hospital. She was an HHMI Investigator at Brigham and Women's Hospital from 1993 to 2007. She has identified many genes and enzymes involved in epigenetic regulation in the fruit fly. In addition, her research has shown the importance of epigenetics in cancer. Her laboratory has identified chromatin remodeling signals and processes that predispose cells to be transformed into cancer cells.
Spiroplasma poulsonii are bacteria of the genus Spiroplasma that are commonly endosymbionts of flies. These bacteria live in the hemolymph of the flies, where they can act as reproductive manipulators or defensive symbionts.
Asifa Akhtar is a Pakistani biologist who has made significant contributions to the field of chromosome regulation. She is senior group leader and director of the Department of Chromatin Regulation at the Max Planck Institute of Immunobiology and Epigenetics. Akhtar was awarded EMBO membership in 2013. She became the first international and female vice president of the Max Planck Society's Biology and Medicine Section in July 2020.

Sex-lethal (Sxl) is a gene found in Dipteran insects, named for its mutation phenotype in Drosophila melanogaster. It is most closely related to the ELAV/HUD subfamily of splicing factors.
X chromosome reactivation (XCR) is the process by which the inactive X chromosome (the Xi) is re-activated in the cells of eutherian female mammals. Therian female mammalian cells have two X chromosomes, while males have only one, requiring X-chromosome inactivation (XCI) for sex-chromosome dosage compensation. In eutherians, XCI is the random inactivation of one of the X chromosomes, silencing its expression. Much of the scientific knowledge currently known about XCR comes from research limited to mouse models or stem cells.











