
A pharmacist, also known as a chemist in Commonwealth English, is a healthcare professional who is knowledgeable about preparation, mechanism of action, clinical usage and legislation of medications in order to dispense them safely to the public and to provide consultancy services. A pharmacist also often serves as a primary care provider in the community and offers services, such as health screenings and immunizations.

Pharmacy is the science and practice of discovering, producing, preparing, dispensing, reviewing and monitoring medications, aiming to ensure the safe, effective, and affordable use of medicines. It is a miscellaneous science as it links health sciences with pharmaceutical sciences and natural sciences. The professional practice is becoming more clinically oriented as most of the drugs are now manufactured by pharmaceutical industries. Based on the setting, pharmacy practice is either classified as community or institutional pharmacy. Providing direct patient care in the community of institutional pharmacies is considered clinical pharmacy.
Therapeutic drug monitoring (TDM) is a branch of clinical chemistry and clinical pharmacology that specializes in the measurement of medication levels in blood. Its main focus is on drugs with a narrow therapeutic range, i.e. drugs that can easily be under- or overdosed. TDM aimed at improving patient care by individually adjusting the dose of drugs for which clinical experience or clinical trials have shown it improved outcome in the general or special populations. It can be based on a a priori pharmacogenetic, demographic and clinical information, and/or on the a posteriori measurement of blood concentrations of drugs (pharmacokinetic monitoring) or biological surrogate or end-point markers of effect (pharmacodynamic monitoring).
Polypharmacy (polypragmasia) is an umbrella term to describe the simultaneous use of multiple medicines by a patient for their conditions. The term polypharmacy is often defined as regularly taking five or more medicines but there is no standard definition and the term has also been used in the context of when a person is prescribed 2 or more medications at the same time. Polypharmacy may be the consequence of having multiple long-term conditions, also known as multimorbidity and is more common in people who are older. In some cases, an excessive number of medications at the same time is worrisome, especially for people who are older with many chronic health conditions, because this increases the risk of an adverse event in that population. In many cases, polypharmacy cannot be avoided, but 'appropriate polypharmacy' practices are encouraged to decrease the risk of adverse effects. Appropriate polypharmacy is defined as the practice of prescribing for a person who has multiple conditions or complex health needs by ensuring that medications prescribed are optimized and follow 'best evidence' practices.
An adverse drug reaction (ADR) is a harmful, unintended result caused by taking medication. ADRs may occur following a single dose or prolonged administration of a drug or may result from the combination of two or more drugs. The meaning of this term differs from the term "side effect" because side effects can be beneficial as well as detrimental. The study of ADRs is the concern of the field known as pharmacovigilance. An adverse event (AE) refers to any unexpected and inappropriate occurrence at the time a drug is used, whether or not the event is associated with the administration of the drug. An ADR is a special type of AE in which a causative relationship can be shown. ADRs are only one type of medication-related harm. Another type of medication-related harm type includes not taking prescribed medications, known as non-adherence. Non-adherence to medications can lead to death and other negative outcomes. Adverse drug reactions require the use of a medication.
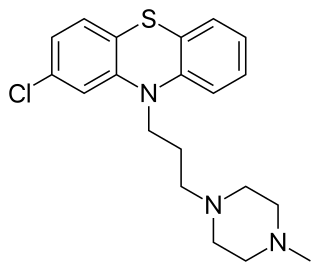
Prochlorperazine, formerly sold under the brand name Compazine among others, is a medication used to treat nausea, migraines, schizophrenia, psychosis and anxiety. It is a less preferred medication for anxiety. It may be taken by mouth, rectally, injection into a vein, or injection into a muscle.
The rebound effect, or rebound phenomenon, is the emergence or re-emergence of symptoms that were either absent or controlled while taking a medication, but appear when that same medication is discontinued, or reduced in dosage. In the case of re-emergence, the severity of the symptoms is often worse than pretreatment levels.
In medicine, patient compliance describes the degree to which a patient correctly follows medical advice. Most commonly, it refers to medication or drug compliance, but it can also apply to other situations such as medical device use, self care, self-directed exercises, or therapy sessions. Both patient and health-care provider affect compliance, and a positive physician-patient relationship is the most important factor in improving compliance. Access to care plays a role in patient adherence, whereby greater wait times to access care contributing to greater absenteeism. The cost of prescription medication also plays a major role.
Pharmacotherapy, also known as pharmacological therapy or drug therapy, is defined as medical treatment that utilizes one or more pharmaceutical drugs to improve ongoing symptoms, treat the underlying condition, or act as a prevention for other diseases (prophylaxis).

In the field of pharmacy, compounding is preparation of custom medications to fit unique needs of patients that cannot be met with mass-produced products. This may be done, for example, to provide medication in a form easier for a given patient to ingest, or to avoid a non-active ingredient a patient is allergic to, or to provide an exact dose that isn't otherwise available. This kind of patient-specific compounding, according to a prescriber's specifications, is referred to as "traditional" compounding. The nature of patient need for such customization can range from absolute necessity to individual optimality to even preference.
A combination drug or a fixed-dose combination (FDC) is a medicine that includes two or more active ingredients combined in a single dosage form. Terms like "combination drug" or "combination drug product" can be common shorthand for an FDC product, although the latter is more precise if in fact referring to a mass-produced product having a predetermined combination of drugs and respective dosages. And it should also be distinguished from the term "combination product" in medical contexts, which without further specification can refer to products that combine different types of medical products—such as device/drug combinations as opposed to drug/drug combinations. When a combination drug product is a "pill", then it may also be a kind of "polypill" or combopill.

Clinical pharmacy is the branch of pharmacy in which clinical pharmacists provide direct patient care that optimizes the use of medication and promotes health, wellness, and disease prevention. Clinical pharmacists care for patients in all health care settings but the clinical pharmacy movement initially began inside hospitals and clinics. Clinical pharmacists often work in collaboration with physicians, physician assistants, nurse practitioners, and other healthcare professionals. Clinical pharmacists can enter into a formal collaborative practice agreement with another healthcare provider, generally one or more physicians, that allows pharmacists to prescribe medications and order laboratory tests.
Pharmaceutical care is a pharmacy practice model developed in the 1990s that describes patient-centered medication management services performed by pharmacists.
Medication therapy management, generally called medicine use review in the United Kingdom, is a service provided typically by pharmacists, medical affairs, and RWE scientists that aims to improve outcomes by helping people to better understand their health conditions and the medications used to manage them. This includes providing education on the disease state and medications used to treat the disease state, ensuring that medicines are taken correctly, reducing waste due to unused medicines, looking for any side effects, and providing education on how to manage any side effects. The process that can be broken down into five steps: medication therapy review, personal medication record, medication-related action plan, intervention and or referral, and documentation and follow-up.
Automatic Generic Substitution is a proposal by the Department of Health (DH) whereby in January 2010 pharmacists could be obliged to substitute a generic version of a medication even if the prescriber had written the prescription for a specific brand, as part of a new deal on drug pricing.
Psychiatric pharmacy, also known as mental health pharmacy, is the area of clinical pharmacy specializing in the treatment of people with psychiatric illnesses through the use of psychotropic medications. It is a branch of neuropsychiatric pharmacy, which includes neurologic pharmacy. Areas where psychiatric pharmacists are found most abundantly are in chemical dependency, developmental disabilities, long-term care facilities, adherence clinics, mental health clinics, and within the prison system. However, psychiatry and neurology are not the only areas where psychiatric pharmacists require comprehensive knowledge. They must also be proficient in clinical problem solving, interprofessionalism, and communication with understanding and empathy for the patient population they serve, as they are a sensitive group.
Pharmacocybernetics is an upcoming field that describes the science of supporting drugs and medications use through the application and evaluation of informatics and internet technologies, so as to improve the pharmaceutical care of patients. It is an interdisciplinary field that integrates the domains of medicine and pharmacy, computer sciences and psychological sciences to design, develop, apply and evaluate technological innovations which improve drugs and medications management, as well as prevent or solve drug-related problems.
Comprehensive medication management (CMM) is the process of delivering clinical services aimed at ensuring a patient's medications (including prescribed, over-the-counter, vitamins, supplements and alternative) are individually assessed to determine that they have an appropriate reason for use, are efficacious for treating their respective medical condition or helping meet defined patient or clinical goals, are safe considering comorbidities and other medications being taken, and are able to be taken by the patient as intended without difficulty.

Drug utilization review refers to a review of prescribing, dispensing, administering and ingesting of medication. This authorized, structured and ongoing review is related to pharmacy benefit managers. Drug use/ utilization evaluation and medication utilization evaluations are the same as drug utilization review.
In pharmacology, augmented renal clearance (ARC) is a phenomenon where certain critically ill patients may display increased clearance of a medication through the kidneys. In many cases, it is observed as a measured creatinine clearance above that which is expected given the patient's age, gender, and other factors. The phenomenon is most commonly observed in patients with neurologic damage, sepsis, major trauma, or burns.







