Related Research Articles
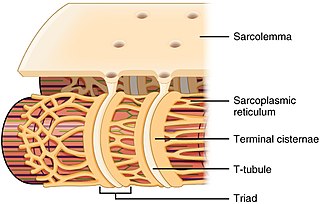
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.

Cardiac muscle is one of three types of vertebrate muscle tissues, the others being skeletal muscle and smooth muscle. It is an involuntary, striated muscle that constitutes the main tissue of the wall of the heart. The cardiac muscle (myocardium) forms a thick middle layer between the outer layer of the heart wall and the inner layer, with blood supplied via the coronary circulation. It is composed of individual cardiac muscle cells joined by intercalated discs, and encased by collagen fibers and other substances that form the extracellular matrix.

Muscle contraction is the activation of tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in muscle length, such as when holding something heavy in the same position. The termination of muscle contraction is followed by muscle relaxation, which is a return of the muscle fibers to their low tension-generating state.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
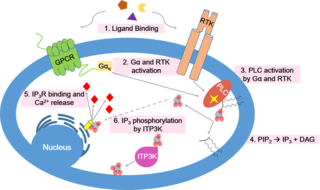
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.

T-tubules are extensions of the cell membrane that penetrate into the center of skeletal and cardiac muscle cells. With membranes that contain large concentrations of ion channels, transporters, and pumps, T-tubules permit rapid transmission of the action potential into the cell, and also play an important role in regulating cellular calcium concentration.
Calcium-induced calcium release (CICR) describes a biological process whereby calcium is able to activate calcium release from intracellular Ca2+ stores (e.g., endoplasmic reticulum or sarcoplasmic reticulum). Although CICR was first proposed for skeletal muscle in the 1970s, it is now known that CICR is unlikely to be the primary mechanism for activating SR calcium release. Instead, CICR is thought to be crucial for excitation-contraction coupling in cardiac muscle. It is now obvious that CICR is a widely occurring cellular signaling process present even in many non-muscle cells, such as in the insulin-secreting pancreatic beta cells, epithelium, and many other cells. Since CICR is a positive-feedback system, it has been of great interest to elucidate the mechanism(s) responsible for its termination.
Calsequestrin is a calcium-binding protein that acts as a calcium buffer within the sarcoplasmic reticulum. The protein helps hold calcium in the cisterna of the sarcoplasmic reticulum after a muscle contraction, even though the concentration of calcium in the sarcoplasmic reticulum is much higher than in the cytosol. It also helps the sarcoplasmic reticulum store an extraordinarily high amount of calcium ions. Each molecule of calsequestrin can bind 18 to 50 Ca2+ ions. Sequence analysis has suggested that calcium is not bound in distinct pockets via EF-hand motifs, but rather via presentation of a charged protein surface. Two forms of calsequestrin have been identified. The cardiac form Calsequestrin-2 (CASQ2) is present in cardiac and slow skeletal muscle and the fast skeletal form Calsequestrin-1(CASQ1) is found in fast skeletal muscle. The release of calsequestrin-bound calcium (through a calcium release channel) triggers muscle contraction. The active protein is not highly structured, more than 50% of it adopting a random coil conformation. When calcium binds there is a structural change whereby the alpha-helical content of the protein increases from 3 to 11%. Both forms of calsequestrin are phosphorylated by casein kinase 2, but the cardiac form is phosphorylated more rapidly and to a higher degree. Calsequestrin is also secreted in the gut where it deprives bacteria of calcium ions..
The sodium-calcium exchanger (often denoted Na+/Ca2+ exchanger, exchange protein, or NCX) is an antiporter membrane protein that removes calcium from cells. It uses the energy that is stored in the electrochemical gradient of sodium (Na+) by allowing Na+ to flow down its gradient across the plasma membrane in exchange for the countertransport of calcium ions (Ca2+). A single calcium ion is exported for the import of three sodium ions. The exchanger exists in many different cell types and animal species. The NCX is considered one of the most important cellular mechanisms for removing Ca2+.

Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited genetic disorder that predisposes those affected to potentially life-threatening abnormal heart rhythms or arrhythmias. The arrhythmias seen in CPVT typically occur during exercise or at times of emotional stress, and classically take the form of bidirectional ventricular tachycardia or ventricular fibrillation. Those affected may be asymptomatic, but they may also experience blackouts or even sudden cardiac death.
A calcium spark is the microscopic release of calcium (Ca2+) from a store known as the sarcoplasmic reticulum (SR), located within muscle cells. This release occurs through an ion channel within the membrane of the SR, known as a ryanodine receptor (RyR), which opens upon activation. This process is important as it helps to maintain Ca2+ concentration within the cell. It also initiates muscle contraction in skeletal and cardiac muscles and muscle relaxation in smooth muscles. Ca2+ sparks are important in physiology as they show how Ca2+ can be used at a subcellular level, to signal both local changes, known as local control, as well as whole cell changes.
Within the muscle tissue of animals and humans, contraction and relaxation of the muscle cells (myocytes) is a highly regulated and rhythmic process. In cardiomyocytes, or cardiac muscle cells, muscular contraction takes place due to movement at a structure referred to as the diad, sometimes spelled "dyad." The dyad is the connection of transverse- tubules (t-tubules) and the junctional sarcoplasmic reticulum (jSR). Like skeletal muscle contractions, Calcium (Ca2+) ions are required for polarization and depolarization through a voltage-gated calcium channel. The rapid influx of calcium into the cell signals for the cells to contract. When the calcium intake travels through an entire muscle, it will trigger a united muscular contraction. This process is known as excitation-contraction coupling. This contraction pushes blood inside the heart and from the heart to other regions of the body.
The Bowditch effect, also known as the Treppe phenomenon or Treppe effect or Staircase Phenomenon, is an autoregulation method by which myocardial tension increases with an increase in heart rate. It was first observed by Henry Pickering Bowditch in 1871.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.

Triadin, also known as TRDN, is a human gene associated with the release of calcium ions from the sarcoplasmic reticulum triggering muscular contraction through calcium-induced calcium release. Triadin is a multiprotein family, arising from different processing of the TRDN gene on chromosome 6. It is a transmembrane protein on the sarcoplasmic reticulum due to a well defined hydrophobic section and it forms a quaternary complex with the cardiac ryanodine receptor (RYR2), calsequestrin (CASQ2) and junctin proteins. The luminal (inner compartment of the sarcoplasmic reticulum) section of Triadin has areas of highly charged amino acid residues that act as luminal Ca2+ receptors. Triadin is also able to sense luminal Ca2+ concentrations by mediating interactions between RYR2 and CASQ2. Triadin has several different forms; Trisk 95 and Trisk 51, which are expressed in skeletal muscle, and Trisk 32 (CT1), which is mainly expressed in cardiac muscle.

Junctophilin 2, also known as JPH2, is a protein which in humans is encoded by the JPH2 gene. Alternative splicing has been observed at this locus and two variants encoding distinct isoforms are described.
JTV-519 (K201) is a 1,4-benzothiazepine derivative that interacts with many cellular targets. It has many structural similarities to diltiazem, a Ca2+ channel blocker used for treatment of hypertension, angina pectoris and some types of arrhythmias. JTV-519 acts in the sarcoplasmic reticulum (SR) of cardiac myocytes by binding to and stabilizing the ryanodine receptor (RyR2) in its closed state. It can be used in the treatment of cardiac arrhythmias, heart failure, catecholaminergic polymorphic ventricular tachycardia (CPVT) and store overload-induced Ca2+ release (SOICR). Currently, this drug has only been tested on animals and its side effects are still unknown. As research continues, some studies have also found a dose-dependent response; where there is no improvement seen in failing hearts at 0.3 μM and a decline in response at 1 μM.

Istaroxime is an investigational drug under development for treatment of acute decompensated heart failure
Calcium buffering describes the processes which help stabilise the concentration of free calcium ions within cells, in a similar manner to how pH buffers maintain a stable concentration of hydrogen ions. The majority of calcium ions within the cell are bound to intracellular proteins, leaving a minority freely dissociated. When calcium is added to or removed from the cytoplasm by transport across the cell membrane or sarcoplasmic reticulum, calcium buffers minimise the effect on changes in cytoplasmic free calcium concentration by binding calcium to or releasing calcium from intracellular proteins. As a result, 99% of the calcium added to the cytosol of a cardiomyocyte during each cardiac cycle becomes bound to calcium buffers, creating a relatively small change in free calcium.
Cardiac excitation-contraction coupling (CardiacEC coupling) describes the series of events, from the production of an electrical impulse (action potential) to the contraction of muscles in the heart. This process is of vital importance as it allows for the heart to beat in a controlled manner, without the need for conscious input. EC coupling results in the sequential contraction of the heart muscles that allows blood to be pumped, first to the lungs (pulmonary circulation) and then around the rest of the body (systemic circulation) at a rate between 60 and 100 beats every minute, when the body is at rest. This rate can be altered, however, by nerves that work to either increase heart rate (sympathetic nerves) or decrease it (parasympathetic nerves), as the body's oxygen demands change. Ultimately, muscle contraction revolves around a charged atom (ion), calcium (Ca2+), which is responsible for converting the electrical energy of the action potential into mechanical energy (contraction) of the muscle. This is achieved in a region of the muscle cell, called the transverse tubule during a process known as calcium induced calcium release.
References
- ↑ Koh; Srinivasan; Ching; Levchenko (2006). "A 3D Monte Carlo Analysis of the Role of Dyadic Space Geometry in Spark Generation". Biophysical Journal. 90 (6). Elsevier: 1999–2014. Bibcode:2006BpJ....90.1999K. doi:10.1529/biophysj.105.065466. PMC 1386779 . PMID 16387773.
- ↑ Page; Buecker (1981). "Development of Dyadic Junctional Complexes between Sarcoplasmic Reticulum and Plasmalemma in Rabbit Left Ventricular Myocardial Cells". Circulation Research. 48 (4): 519–522. doi: 10.1161/01.RES.48.4.519 . PMID 7460221.
- ↑ Cannell; Kong; Imtiaz; Laver (2013). "Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination". Biophysical Journal. 104 (10). Elsevier: 2149–59. Bibcode:2013BpJ...104.2149C. doi:10.1016/j.bpj.2013.03.058. PMC 3660628 . PMID 23708355.