Related Research Articles

Dermatitis is inflammation of the skin, typically characterized by itchiness, redness and a rash. In cases of short duration, there may be small blisters, while in long-term cases the skin may become thickened. The area of skin involved can vary from small to covering the entire body. Dermatitis is often called eczema, and the difference between those terms is not standardized.

Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by patches of abnormal skin. These areas are red, pink, or purple, dry, itchy, and scaly. Psoriasis varies in severity from small localized patches to complete body coverage. Injury to the skin can trigger psoriatic skin changes at that spot, which is known as the Koebner phenomenon.

Pimecrolimus is an immunomodulating agent of the calcineurin inhibitor class used in the treatment of atopic dermatitis (eczema). It is available as a topical cream, once marketed by Novartis under the trade name Elidel.

Nummular dermatitis is one of the many forms of dermatitis. It is characterized by round or oval-shaped itchy lesions. The name comes from the Latin word "nummus," which means "coin."
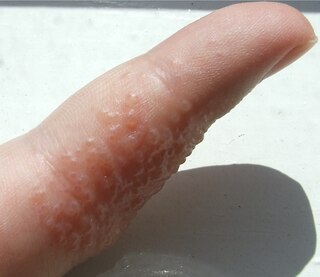
Dyshidrosis is a type of dermatitis that is characterized by itchy blisters on the palms of the hands and bottoms of the feet. Blisters are generally one to two millimeters in size and heal over three weeks. However, they often recur. Redness is not usually present. Repeated attacks may result in fissures and skin thickening.

Desonide (INN) is a low-potency topical corticosteroid anti-inflammatory that has been available since the 1970s. It is primarily used to treat atopic dermatitis (eczema), seborrheic dermatitis, contact dermatitis and psoriasis in both adults and children. It has a fairly good safety profile and is available as a cream, ointment, lotion, and as a foam under the tradename Verdeso Foam. Other trade names for creams, lotions, and ointments include Tridesilon, DesOwen, Desonate. It is a group VI corticosteroid under US classification, the second least potent group.

Atopic dermatitis (AD), also known as atopic eczema, is a long-term type of inflammation of the skin (dermatitis). It results in itchy, red, swollen, and cracked skin. Clear fluid may come from the affected areas, which can thicken over time. AD may also simply be called eczema, a term that generally refers to a larger group of skin conditions.
The SCORAD index is a clinical tool for assessing the extent of the disease, disease intensity, and subjective symptoms of atopic dermatitis. It gives approximate weights of 60% to intensity and 20% each to extent and subjective symptoms. These are used to calculate a maximum total score of 103, however, the scores for each category can be used individually if clinically appropriate.
Hypnodermatology is an informal label for the use of hypnosis in treating the skin conditions that fall between conventional medical dermatology and the mental health disciplines.
Psoriasis Area and Severity Index (PASI) is the most widely used tool for the measurement of severity of psoriasis. PASI combines the assessment of the severity of lesions and the area affected into a single score in the range 0 to 72.
In medicine, a finger tip unit (FTU) is defined as the amount of ointment, cream or other semi-solid dosage form expressed from a tube with a 5 mm diameter nozzle, applied from the distal skin-crease to the tip of the index finger of an adult. The "distal skin-crease" is the skin crease over the joint nearest the end of the finger. One FTU is enough to treat an area of skin twice the size of the flat of an adult's hand with the fingers together, i.e. a "handprint". Two FTUs are approximately equivalent to 1 g of topical steroid.
Topical steroids are the topical forms of corticosteroids. Topical steroids are the most commonly prescribed topical medications for the treatment of rash and eczema. Topical steroids have anti-inflammatory properties and are classified based on their skin vasoconstrictive abilities. There are numerous topical steroid products. All the preparations in each class have the same anti-inflammatory properties but essentially differ in base and price.
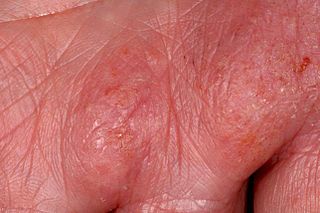
Hand eczema presents on the palms and soles, and may sometimes be difficult or impossible to differentiate from atopic dermatitis, allergic contact dermatitis, and psoriasis, which also commonly involve the hands. Even a biopsy of all these conditions may not result in a definitive diagnosis, as all three conditions may demonstrate spongiosis and crusting on the hands.
The Quality of Life Index for Atopic Dermatitis (QoLIAD) is a disease specific patient reported outcome which measures the impact that atopic dermatitis (AD) has on a given patient's quality of life.

Crisaborole, sold under the brand name Eucrisa among others, is a nonsteroidal topical medication used for the treatment of mild-to-moderate atopic dermatitis (eczema) in adults and children.

Calcipotriol/betamethasone dipropionate, sold under the brand name Taclonex among others, is a fixed-dose combination medication of the synthetic vitamin D3 analog calcipotriol (also known as calcipotriene) and the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis. It is used in the form of ointment, topical suspension, gel, aerosol, and foam.
The Dermatology life Quality Index (DLQI) is a ten-question questionnaire used to measure the impact of skin disease on the quality of life of an affected person. It is designed for people aged 16 years and above.
Sodium hypochlorite washes are skin cleansers formulated with sodium hypochlorite (NaOCl) and surfactants. These cleansing liquids or gels are lathered onto wet skin and rinsed off. They are recommended for inflammatory skin conditions, microbial driven skin disorders and body odor.

Abrocitinib, sold under the brand name Cibinqo, is a medication used for the treatment of atopic dermatitis (eczema). It is a Janus kinase inhibitor and it was developed by Pfizer. It is taken by mouth.

Topical glucocorticoids are the topical forms of glucocorticoids. Topical glucocorticoids are used in the treatment of many skin conditions. They provide anti-inflammatory, antimitotic, and immune-system suppressing actions through various mechanisms.
References
- ↑ Hanifin, J. M.; Thurston, M.; Omoto, M.; Cherill, R.; Tofte, S. J.; Graeber, M. (2001). "The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group". Experimental Dermatology. 10 (1): 11–18. doi:10.1034/j.1600-0625.2001.100102.x. PMID 11168575. S2CID 25864663.
- ↑ Leshem, Y. A.; Hajar, T; Hanifin, J. M.; Simpson, E. L. (2015). "What the Eczema Area and Severity Index score tells us about the severity of atopic dermatitis: An interpretability study". British Journal of Dermatology. 172 (5): 1353–1357. doi:10.1111/bjd.13662. PMID 25580670. S2CID 207072864.
- ↑ Tofte, Susan J.; Graeber, Michael; Cherill, Robert J.; Omoto, Mitsuyoshi; Thurston, Mark; Hanifin, Jon (1 September 1998). "Eczema area and severity index (EASI): A new tool to evaluate atopic dermatitis". Journal of the European Academy of Dermatology and Venereology. 11 (Supplement 2): S197. doi:10.1016/S0926-9959(98)95291-6. ISSN 0926-9959. S2CID 72210425.
- ↑ Schmitt, Jochen; Spuls, Phyllis I.; Thomas, Kim S.; Simpson, Eric; Furue, Masutaka; Deckert, Stefanie; Dohil, Magdalene; Apfelbacher, Christian; Singh, Jasvinder A.; Chalmers, Joanne; Williams, Hywel C. (October 2014). "The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials". Journal of Allergy and Clinical Immunology. 134 (4): 800–807. doi: 10.1016/j.jaci.2014.07.043 . ISSN 0091-6749. PMID 25282560.
- ↑ Hanifin, Jon M.; Baghoomian, Wenelia; Grinich, Erin; Leshem, Yael A.; Jacobson, Michael; Simpson, Eric Lawrence (2022). "The Eczema Area and Severity Index—A Practical Guide". Dermatitis. 33 (3): 187–192. doi:10.1097/DER.0000000000000895. PMC 9154300 . PMID 35594457.