
In chemistry and manufacturing, electrolysis is a technique that uses direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity".

A sensor is a device that produces an output signal for the purpose of sensing a physical phenomenon.

An electro-galvanic fuel cell is an electrochemical device which consumes a fuel to produce an electrical output by a chemical reaction. One form of electro-galvanic fuel cell based on the oxidation of lead is commonly used to measure the concentration of oxygen gas in underwater diving and medical breathing gases.
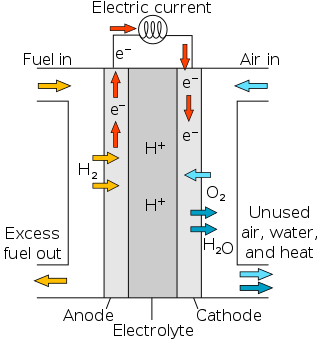
Proton-exchange membrane fuel cells (PEMFC), also known as polymer electrolyte membrane (PEM) fuel cells, are a type of fuel cell being developed mainly for transport applications, as well as for stationary fuel-cell applications and portable fuel-cell applications. Their distinguishing features include lower temperature/pressure ranges and a special proton-conducting polymer electrolyte membrane. PEMFCs generate electricity and operate on the opposite principle to PEM electrolysis, which consumes electricity. They are a leading candidate to replace the aging alkaline fuel-cell technology, which was used in the Space Shuttle.
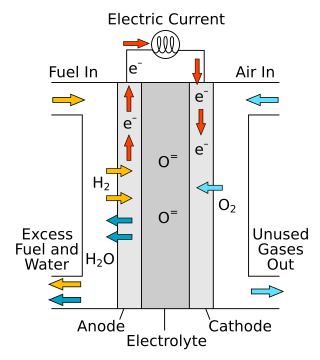
A solid oxide fuel cell is an electrochemical conversion device that produces electricity directly from oxidizing a fuel. Fuel cells are characterized by their electrolyte material; the SOFC has a solid oxide or ceramic electrolyte.

The alkaline fuel cell (AFC), also known as the Bacon fuel cell after its British inventor, Francis Thomas Bacon, is one of the most developed fuel cell technologies. Alkaline fuel cells consume hydrogen and pure oxygen, to produce potable water, heat, and electricity. They are among the most efficient fuel cells, having the potential to reach 70%.
An oxygen sensor (or lambda sensor, where lambda refers to air–fuel equivalence ratio, usually denoted by λ) or probe or sond, is an electronic device that measures the proportion of oxygen (O2) in the gas or liquid being analysed.

In electrochemistry, cyclic voltammetry (CV) is a type of potentiodynamic measurement. In a cyclic voltammetry experiment, the working electrode potential is ramped linearly versus time. Unlike in linear sweep voltammetry, after the set potential is reached in a CV experiment, the working electrode's potential is ramped in the opposite direction to return to the initial potential. These cycles of ramps in potential may be repeated as many times as needed. The current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. Cyclic voltammetry is generally used to study the electrochemical properties of an analyte in solution or of a molecule that is adsorbed onto the electrode.
A nitrogen oxide sensor or NOx sensor is typically a high-temperature device built to detect nitrogen oxides in combustion environments such as an automobile, truck tailpipe or smokestack.

Voltammetry is a category of electroanalytical methods used in analytical chemistry and various industrial processes. In voltammetry, information about an analyte is obtained by measuring the current as the potential is varied. The analytical data for a voltammetric experiment comes in the form of a voltammogram which plots the current produced by the analyte versus the potential of the working electrode.

A carbon monoxide detector or CO detector is a device that detects the presence of the carbon monoxide (CO) gas to prevent carbon monoxide poisoning. In the late 1990s Underwriters Laboratories changed the definition of a single station CO detector with a sound device to carbon monoxide (CO) alarm. This applies to all CO safety alarms that meet UL 2034 standard; however for passive indicators and system devices that meet UL 2075, UL refers to these as carbon monoxide detectors.
A gas detector is a device that detects the presence of gases in an area, often as part of a safety system. A gas detector can sound an alarm to operators in the area where the leak is occurring, giving them the opportunity to leave. This type of device is important because there are many gases that can be harmful to organic life, such as humans or animals.
Gas diffusion electrodes (GDE) are electrodes with a conjunction of a solid, liquid and gaseous interface, and an electrical conducting catalyst supporting an electrochemical reaction between the liquid and the gaseous phase.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
The electrochemical reduction of carbon dioxide, also known as electrolysis of carbon dioxide, is the conversion of carbon dioxide to more reduced chemical species using electrical energy. It is one possible step in the broad scheme of carbon capture and utilization, nevertheless it is deemed to be one of the most promising approaches.
A biotransducer is the recognition-transduction component of a biosensor system. It consists of two intimately coupled parts; a bio-recognition layer and a physicochemical transducer, which acting together converts a biochemical signal to an electronic or optical signal. The bio-recognition layer typically contains an enzyme or another binding protein such as antibody. However, oligonucleotide sequences, sub-cellular fragments such as organelles and receptor carrying fragments, single whole cells, small numbers of cells on synthetic scaffolds, or thin slices of animal or plant tissues, may also comprise the bio-recognition layer. It gives the biosensor selectivity and specificity. The physicochemical transducer is typically in intimate and controlled contact with the recognition layer. As a result of the presence and biochemical action of the analyte, a physico-chemical change is produced within the biorecognition layer that is measured by the physicochemical transducer producing a signal that is proportionate to the concentration of the analyte. The physicochemical transducer may be electrochemical, optical, electronic, gravimetric, pyroelectric or piezoelectric. Based on the type of biotransducer, biosensors can be classified as shown to the right.

Electrochemical stripping analysis is a set of analytical chemistry methods based on voltammetry or potentiometry that are used for quantitative determination of ions in solution. Stripping voltammetry have been employed for analysis of organic molecules as well as metal ions. Carbon paste, glassy carbon paste, and glassy carbon electrodes when modified are termed as chemically modified electrodes and have been employed for the analysis of organic and inorganic compounds.
Gas-diffusion electrocrystallization (GDEx) is an electrochemical process consisting on the reactive precipitation of metal ions in solution with intermediaries produced by the electrochemical reduction of gases, at gas diffusion electrodes. It can serve for the recovery of metals or metalloids into solid precipitates or for the synthesis of libraries of nanoparticles.
Workplace exposure monitoring is the monitoring of substances in a workplace that are chemical or biological hazards. It is performed in the context of workplace exposure assessment and risk assessment. Exposure monitoring analyzes hazardous substances in the air or on surfaces of a workplace, and is complementary to biomonitoring, which instead analyzes toxicants or their effects within workers.









