Related Research Articles

A gametophyte is one of the two alternating multicellular phases in the life cycles of plants and algae. It is a haploid multicellular organism that develops from a haploid spore that has one set of chromosomes. The gametophyte is the sexual phase in the life cycle of plants and algae. It develops sex organs that produce gametes, haploid sex cells that participate in fertilization to form a diploid zygote which has a double set of chromosomes. Cell division of the zygote results in a new diploid multicellular organism, the second stage in the life cycle known as the sporophyte. The sporophyte can produce haploid spores by meiosis that on germination produce a new generation of gametophytes.

An embryo is an initial stage of development of a multicellular organism. In organisms that reproduce sexually, embryonic development is the part of the life cycle that begins just after fertilization of the female egg cell by the male sperm cell. The resulting fusion of these two cells produces a single-celled zygote that undergoes many cell divisions that produce cells known as blastomeres. The blastomeres are arranged as a solid ball that when reaching a certain size, called a morula, takes in fluid to create a cavity called a blastocoel. The structure is then termed a blastula, or a blastocyst in mammals.

In botany, a seed is a plant embryo and food reserve enclosed in a protective outer covering called a seed coat (testa). More generally, the term "seed" means anything that can be sown, which may include seed and husk or tuber. Seeds are the product of the ripened ovule, after the embryo sac is fertilized by sperm from pollen, forming a zygote. The embryo within a seed develops from the zygote and grows within the mother plant to a certain size before growth is halted.

Fertilisation or fertilization, also known as generative fertilisation, syngamy and impregnation, is the fusion of gametes to give rise to a zygote and initiate its development into a new individual organism or offspring. While processes such as insemination or pollination, which happen before the fusion of gametes, are also sometimes informally referred to as fertilisation, these are technically separate processes. The cycle of fertilisation and development of new individuals is called sexual reproduction. During double fertilisation in angiosperms, the haploid male gamete combines with two haploid polar nuclei to form a triploid primary endosperm nucleus by the process of vegetative fertilisation.

The egg cell or ovum is the female reproductive cell, or gamete, in most anisogamous organisms. The term is used when the female gamete is not capable of movement (non-motile). If the male gamete (sperm) is capable of movement, the type of sexual reproduction is also classified as oogamous. A nonmotile female gamete formed in the oogonium of some algae, fungi, oomycetes, or bryophytes is an oosphere. When fertilized, the oosphere becomes the oospore.

A pollen tube is a tubular structure produced by the male gametophyte of seed plants when it germinates. Pollen tube elongation is an integral stage in the plant life cycle. The pollen tube acts as a conduit to transport the male gamete cells from the pollen grain—either from the stigma to the ovules at the base of the pistil or directly through ovule tissue in some gymnosperms. In maize, this single cell can grow longer than 12 inches (30 cm) to traverse the length of the pistil.

In seed plants, the ovule is the structure that gives rise to and contains the female reproductive cells. It consists of three parts: the integument, forming its outer layer, the nucellus, and the female gametophyte in its center. The female gametophyte — specifically termed a megagametophyte— is also called the embryo sac in angiosperms. The megagametophyte produces an egg cell for the purpose of fertilization. The ovule is a small structure present in the ovary. It is attached to the placenta by a stalk called a funicle. The funicle provides nourishment to the ovule.

The endosperm is a tissue produced inside the seeds of most of the flowering plants following double fertilization. It is triploid in most species, which may be auxin-driven. It surrounds the embryo and provides nutrition in the form of starch, though it can also contain oils and protein. This can make endosperm a source of nutrition in animal diet. For example, wheat endosperm is ground into flour for bread, while barley endosperm is the main source of sugars for beer production. Other examples of endosperm that forms the bulk of the edible portion are coconut "meat" and coconut "water", and corn. Some plants, such as orchids, lack endosperm in their seeds.

Plant callus is a growing mass of unorganized plant parenchyma cells. In living plants, callus cells are those cells that cover a plant wound. In biological research and biotechnology callus formation is induced from plant tissue samples (explants) after surface sterilization and plating onto tissue culture medium in vitro. The culture medium is supplemented with plant growth regulators, such as auxin, cytokinin, and gibberellin, to initiate callus formation or somatic embryogenesis. Callus initiation has been described for all major groups of land plants.
Plant embryonic development, also plant embryogenesis is a process that occurs after the fertilization of an ovule to produce a fully developed plant embryo. This is a pertinent stage in the plant life cycle that is followed by dormancy and germination. The zygote produced after fertilization must undergo various cellular divisions and differentiations to become a mature embryo. An end stage embryo has five major components including the shoot apical meristem, hypocotyl, root meristem, root cap, and cotyledons. Unlike the embryonic development in animals, and specifically in humans, plant embryonic development results in an immature form of the plant, lacking most structures like leaves, stems, and reproductive structures. However, both plants and animals including humans, pass through a phylotypic stage that evolved independently and that causes a developmental constraint limiting morphological diversification.
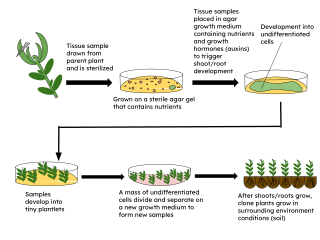
Micropropagation or tissue culture is the practice of rapidly multiplying plant stock material to produce many progeny plants, using modern plant tissue culture methods.

Gynoecium is most commonly used as a collective term for the parts of a flower that produce ovules and ultimately develop into the fruit and seeds. The gynoecium is the innermost whorl of a flower; it consists of pistils and is typically surrounded by the pollen-producing reproductive organs, the stamens, collectively called the androecium. The gynoecium is often referred to as the "female" portion of the flower, although rather than directly producing female gametes, the gynoecium produces megaspores, each of which develops into a female gametophyte which then produces egg cells.
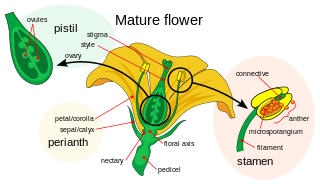
Double fertilization is a complex fertilization mechanism of flowering plants (angiosperms). This process involves the joining of a female gametophyte with two male gametes (sperm). It begins when a pollen grain adheres to the stigma of the carpel, the female reproductive structure of a flower. The pollen grain then takes in moisture and begins to germinate, forming a pollen tube that extends down toward the ovary through the style. The tip of the pollen tube then enters the ovary and penetrates through the micropyle opening in the ovule. The pollen tube proceeds to release the two sperm in the embryo sacs.

Placentation refers to the formation, type and structure, or arrangement of the placenta. The function of placentation is to transfer nutrients, respiratory gases, and water from maternal tissue to a growing embryo, and in some instances to remove waste from the embryo. Placentation is best known in live-bearing mammals (theria), but also occurs in some fish, reptiles, amphibians, a diversity of invertebrates, and flowering plants. In vertebrates, placentas have evolved more than 100 times independently, with the majority of these instances occurring in squamate reptiles.
A seedless fruit is a fruit developed to possess no mature seeds. Since eating seedless fruits is generally easier and more convenient, they are considered commercially valuable.
The mechanisms of reproductive isolation are a collection of evolutionary mechanisms, behaviors and physiological processes critical for speciation. They prevent members of different species from producing offspring, or ensure that any offspring are sterile. These barriers maintain the integrity of a species by reducing gene flow between related species.

Nucellar embryony is a form of seed reproduction that occurs in certain plant species, including many citrus varieties. Nucellar embryony is a type of apomixis, where eventually nucellar embryos from the nucellus tissue of the ovule are formed, independent of meiosis and sexual reproduction. During the development of seeds in plants that possess this genetic trait, the nucellus tissue which surrounds the megagametophyte can produce nucellar cells, also termed initial cells. These additional embryos (polyembryony) are genetically identical to the parent plant, rendering them as clones. By contrast, zygotic seedlings are sexually produced and inherit genetic material from both parents. Most angiosperms reproduce sexually through double fertilization. Different from nucellar embryony, double fertilization occurs via the syngamy of sperm and egg cells, producing a triploid endosperm and a diploid zygotic embryo. In nucellar embryony, embryos are formed asexually from the nucellus tissue. Zygotic and nucellar embryos can occur in the same seed (monoembryony), and a zygotic embryo can divide to produce multiple embryos. The nucellar embryonic initial cells form, divide, and expand. Once the zygotic embryo becomes dominant, the initial cells stop dividing and expanding. Following this stage, the zygotic embryo continues to develop and the initial cells continue to develop as well, forming nucellar embryos. The nucellar embryos generally end up outcompeting the zygotic embryo, rending the zygotic embryo dormant. The polyembryonic seed is then formed by the many adventitious embryos within the ovule. The nucellar embryos produced via apomixis inherit its mother's genetics, making them desirable for citrus propagation, research, and breeding.

Plant tissue culture is a collection of techniques used to maintain or grow plant cells, tissues, or organs under sterile conditions on a nutrient culture medium of known composition. It is widely used to produce clones of a plant in a method known as micropropagation. Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including:

Plant breeding is the science of changing the traits of plants in order to produce desired characteristics. It has been used to improve the quality of nutrition in products for humans and animals. The goals of plant breeding are to produce crop varieties that boast unique and superior traits for a variety of applications. The most frequently addressed agricultural traits are those related to biotic and abiotic stress tolerance, grain or biomass yield, end-use quality characteristics such as taste or the concentrations of specific biological molecules and ease of processing.
Selective embryo abortion, is a form of non-random, premature termination of embryonic development in plants. Selective embryo abortion assumes that embryo termination depends on the genetic quality of seeds developing within an ovary, and predicts that successfully matured seeds will be of greater fitness than aborted seeds. Consequently, selective embryo abortion has the potential to act as a unique stage of natural selection, influencing the evolution of plant populations and species. This concept was described by botanist John T. Buchholz in 1922 under his framework of developmental selection, which referred to selective embryo abortion as “interovular selection.”
References
- ↑ Sage, T.L.; Strumas, F.; Cole, W.W.; Barret,S (2010). "Embryo rescue and plant regeneration following interspecific crosses in the genus Hylocereus (Cactaceae)". Euphytica. 174: 73–82. doi:10.1007/s10681-010-0135-x. S2CID 7837522.
- ↑ Miyajuma, D. (2006). "Ovules that failed to form seeds in zinnia (Zinnia violacec Cav)". Sci Hortic. 107 (2): 176–182. doi:10.1016/j.scienta.2005.06.014.
- ↑ Reed, Sandra (2005). Robert N. Trigiano; Dennis J. Gray (eds.). Plant Development and Biotechnology (PDF). CRC Press. pp. 235–239. ISBN 0-8493-1614-6. Archived from the original (PDF) on 2012-03-12. Retrieved 2011-04-18.
- ↑ Bridgen, Mark P. (1994). "A Review of Plant Embryo Culture". HortScience. 29 (11): 1243–1246. doi: 10.21273/hortsci.29.11.1243 .
- ↑ Sharma, D.R.; Daur, R.; Kumar K. (1996). "Embryo rescue in plants-a review". Euphytica. 89 (3): 325–337. doi:10.1007/BF00022289. S2CID 206766831.
- ↑ Amanate-Bordeos, A.D.; Nelson, R.J.; Oliva, N.P.; Dalmacio, R.D.; Leung, H.; Sitch, L.A. (1992). "Transfer of blast and bacterial blight resistance from the tetrapliod wild rice Oryza minuta to the cultivated rice, O. sativa". Theor. Appl. Genet. 84 (3–4): 345–354. doi:10.1007/bf00229493. PMID 24203194. S2CID 6451011.
- ↑ Mehetre, S. S; Aher, A. R. (2004). "Embryo rescue: A tool to overcome incompatible interspecific hybridization in Gossypium Linn. --A review". Indian Journal of Biotechnology. 3: 29–36.
- ↑ Cisneros, Aroldo; Tel-Zur (2010). "Embryo rescue and plant regeneration following interspecific crosses in the genus Hylocereus (Cactaceae)". Euphytica. 174: 73–82. doi:10.1007/s10681-010-0135-x. S2CID 7837522.
- ↑ Ikeda, N.; Niimi, Y. Han D. (2003). "Production of seedling from ovules excised at the zygote stage in Lilium spp". Plant Cell Tissue Organ Cult. 73 (2): 159–166. doi:10.1023/A:1022818413617. S2CID 7305410.
- ↑ Murashige, T; Skoog, F. (1962). "A revised medium for rapid growth and bioassays with tobacco tissue cultures". Physiol. Plant. 15 (3): 473–497. doi:10.1111/j.1399-3054.1962.tb08052.x. S2CID 84645704.
- ↑ Gamborg, O.L; Miller, R.A.; Ojima, K. (1968). "Nutrient requirements of suspension cultures of soybean root cells". Exp. Cell Res. 50 (1): 151–158 [157]. doi:10.1016/0014-4827(68)90403-5. PMID 5650857.