
In cell biology, the cytoplasm is all of the material within a eukaryotic cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol, the organelles, and various cytoplasmic inclusions. The cytoplasm is about 80% water and is usually colorless.

Biophysics is an interdisciplinary science that applies approaches and methods traditionally used in physics to study biological phenomena. Biophysics covers all scales of biological organization, from molecular to organismic and populations. Biophysical research shares significant overlap with biochemistry, molecular biology, physical chemistry, physiology, nanotechnology, bioengineering, computational biology, biomechanics, developmental biology and systems biology.

The lipid bilayer is a thin polar membrane made of two layers of lipid molecules. These membranes are flat sheets that form a continuous barrier around all cells. The cell membranes of almost all organisms and many viruses are made of a lipid bilayer, as are the nuclear membrane surrounding the cell nucleus, and membranes of the membrane-bound organelles in the cell. The lipid bilayer is the barrier that keeps ions, proteins and other molecules where they are needed and prevents them from diffusing into areas where they should not be. Lipid bilayers are ideally suited to this role, even though they are only a few nanometers in width, because they are impermeable to most water-soluble (hydrophilic) molecules. Bilayers are particularly impermeable to ions, which allows cells to regulate salt concentrations and pH by transporting ions across their membranes using proteins called ion pumps.

The fluid mosaic model explains various observations regarding the structure of functional cell membranes. According to this biological model, there is a lipid bilayer in which protein molecules are embedded. The lipid bilayer gives fluidity and elasticity to the membrane. Small amounts of carbohydrates are also found in the cell membrane. The biological model, which was devised by SJ Singer and G. L. Nicolson in 1972, describes the cell membrane as a two-dimensional liquid that restricts the lateral diffusion of membrane components. Such domains are defined by the existence of regions within the membrane with special lipid and protein cocoon that promote the formation of lipid rafts or protein and glycoprotein complexes. Another way to define membrane domains is the association of the lipid membrane with the cytoskeleton filaments and the extracellular matrix through membrane proteins. The current model describes important features relevant to many cellular processes, including: cell-cell signaling, apoptosis, cell division, membrane budding, and cell fusion. The fluid mosaic model is the most acceptable model of the plasma membrane. Its main function is to separate the contents of the cell from the outside.

The plasma membranes of cells contain combinations of glycosphingolipids, cholesterol and protein receptors organised in glycolipoprotein lipid microdomains termed lipid rafts. Their existence in cellular membranes remains somewhat controversial. It has been proposed that they are specialized membrane microdomains which compartmentalize cellular processes by serving as organising centers for the assembly of signaling molecules, allowing a closer interaction of protein receptors and their effectors to promote kinetically favorable interactions necessary for the signal transduction. Lipid rafts influence membrane fluidity and membrane protein trafficking, thereby regulating neurotransmission and receptor trafficking. Lipid rafts are more ordered and tightly packed than the surrounding bilayer, but float freely within the membrane bilayer. Although more common in the cell membrane, lipid rafts have also been reported in other parts of the cell, such as the Golgi apparatus and lysosomes.
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemical signals and mechanical signals. Errors during this process have serious consequences, including intellectual disability, vascular disease, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells.

Molecular biophysics is a rapidly evolving interdisciplinary area of research that combines concepts in physics, chemistry, engineering, mathematics and biology. It seeks to understand biomolecular systems and explain biological function in terms of molecular structure, structural organization, and dynamic behaviour at various levels of complexity. This discipline covers topics such as the measurement of molecular forces, molecular associations, allosteric interactions, Brownian motion, and cable theory. Additional areas of study can be found on Outline of Biophysics. The discipline has required development of specialized equipment and procedures capable of imaging and manipulating minute living structures, as well as novel experimental approaches.
A cell membrane defines a boundary between a cell and its environment. The primary constituent of a membrane is a phospholipid bilayer that forms in a water-based environment due to the hydrophilic nature of the lipid head and the hydrophobic nature of the two tails. In addition there are other lipids and proteins in the membrane, the latter typically in the form of isolated rafts.
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion of proteins and other bio-molecules within the membrane, there-by affecting the functions of these things.
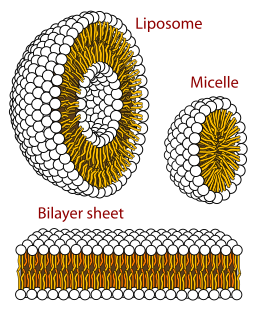
Polymorphism in biophysics is the ability of lipids to aggregate in a variety of ways, giving rise to structures of different shapes, known as "phases". This can be in the form of sphere of lipid molecules (micelles), pairs of layers that face one another, a tubular arrangement (hexagonal), or various cubic phases. More complicated aggregations have also been observed, such as rhombohedral, tetragonal and orthorhombic phases.

Single-particle tracking (SPT) is the observation of the motion of individual particles within a medium. The coordinates time series, which can be either in two dimensions or in three dimensions, is referred to as a trajectory. The trajectory is typically analyzed using statistical methods to extract information about the underlying dynamics of the particle. These dynamics can reveal information about the type of transport being observed, the medium where the particle is moving, and interactions with other particles. In the case of random motion, trajectory analysis can be used to measure the diffusion coefficient.
A model lipid bilayer is any bilayer assembled in vitro, as opposed to the bilayer of natural cell membranes or covering various sub-cellular structures like the nucleus. They are used to study the fundamental properties of biological membranes in a simplified and well-controlled environment, and increasingly in bottom-up synthetic biology for the construction of artificial cells. A model bilayer can be made with either synthetic or natural lipids. The simplest model systems contain only a single pure synthetic lipid. More physiologically relevant model bilayers can be made with mixtures of several synthetic or natural lipids.
Microrheology is a technique used to measure the rheological properties of a medium, such as microviscosity, via the measurement of the trajectory of a flow tracer. It is a new way of doing rheology, traditionally done using a rheometer. There are two types of microrheology: passive microrheology and active microrheology. Passive microrheology uses inherent thermal energy to move the tracers, whereas active microrheology uses externally applied forces, such as from a magnetic field or an optical tweezer, to do so. Microrheology can be further differentiated into 1- and 2-particle methods.

Interference reflection microscopy (IRM) is an optical microscopy technique that utilizes polarized light to form an image of an object on a glass surface. The intensity of the signal is a measure of proximity of the object to the glass surface. This technique can be used to study events at the cell membrane without the use of a (fluorescent) label in contrast to TIRF microscopy.

The cell membrane is a biological membrane that separates the interior of all cells from the outside environment and protects the cell from its environment. The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions and organic molecules. In addition, cell membranes are involved in a variety of cellular processes such as cell adhesion, ion conductivity and cell signalling and serve as the attachment surface for several extracellular structures, including the cell wall and the carbohydrate layer called the glycocalyx, as well as the intracellular network of protein fibers called the cytoskeleton. In the field of synthetic biology, cell membranes can be artificially reassembled.
Force Spectrum Microscopy (FSM) is an application of active microrheology developed to measure aggregate random forces in the cytoplasm. Large, inert flow tracers are injected into live cells and become lodged inside the cytoskeletal mesh, wherein it is oscillated by repercussions from active motor proteins. The magnitude of these random forces can be inferred from the frequency of oscillation of tracer particles. Tracking the fluctuations of tracer particles using optical microscopy can isolate the contribution of active random forces to intracellular molecular transport from that of Brownian motion.
Sarah L. Keller is an American biophysicist, studying problems at the intersection between biology and chemistry. She investigates self-assembling soft matter systems. Her current main research focus is understanding how simple lipid mixtures within bilayer membranes give rise to membrane's complex phase behavior.
P. B. Sunil Kumar is an physicist, professor and director at IIT Palakkad. He also holds professorship(on lien) at Department of Physics, IIT Madras. He is known for his research on Soft matter and Biological Physics. He is an elected member of Kerala Science Congress.

Suliana Manley is an American biophysicist. Her research focuses on the development of high-resolution optical instruments, and their application in studying the organization and dynamics of proteins. She is an associate professor at École Polytechnique Fédérale de Lausanne and heads the Laboratory of Experimental Biophysics.

Joshua Shaevitz is an American biophysicist and Professor of Physics at the Lewis-Sigler Institute at Princeton University in Princeton, NJ. He is known for his work in single-molecule biophysics, bacterial growth and motility, and animal behavior.











