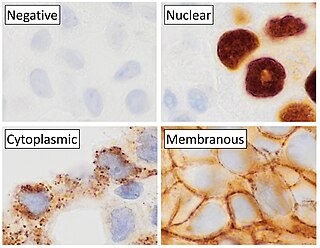
Immunohistochemistry (IHC) is a form of immunostaining. It involves the process of selectively identifying antigens (proteins) in cells and tissue, by exploiting the principle of antibodies binding specifically to antigens in biological tissues. Albert Hewett Coons, Ernest Berliner, Norman Jones and Hugh J Creech was the first to develop immunofluorescence in 1941. This led to the later development of immunohistochemistry.

Selective estrogen receptor modulators (SERMs), also known as estrogen receptor agonist/antagonists (ERAAs), are a class of drugs that act on the estrogen receptor (ER). A characteristic that distinguishes these substances from pure ER agonists and antagonists is that their action is different in various tissues, thereby granting the possibility to selectively inhibit or stimulate estrogen-like action in various tissues.

Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome. Tamoxifen is typically taken daily by mouth for five years for breast cancer.

Estrogen receptors (ERs) are a group of proteins found inside cells. They are receptors that are activated by the hormone estrogen (17β-estradiol). Two classes of ER exist: nuclear estrogen receptors, which are members of the nuclear receptor family of intracellular receptors, and membrane estrogen receptors (mERs), which are mostly G protein-coupled receptors. This article refers to the former (ER).

Receptor tyrosine-protein kinase erbB-2 is a protein that normally resides in the membranes of cells and is encoded by the ERBB2 gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The human protein is also frequently referred to as HER2 or CD340.

Toremifene, sold under the brand name Fareston among others, is a medication which is used in the treatment of advanced breast cancer in postmenopausal women. It is taken by mouth.

Estrogen receptor alpha (ERα), also known as NR3A1, is one of two main types of estrogen receptor, a nuclear receptor that is activated by the sex hormone estrogen. In humans, ERα is encoded by the gene ESR1.

Estrogen receptor beta (ERβ) also known as NR3A2 is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by the ESR2 gene.

Afimoxifene, also known as 4-hydroxytamoxifen (4-OHT) and by its tentative brand name TamoGel, is a selective estrogen receptor modulator (SERM) of the triphenylethylene group and an active metabolite of tamoxifen. The drug is under development under the tentative brand name TamoGel as a topical gel for the treatment of hyperplasia of the breast. It has completed a phase II clinical trial for cyclical mastalgia, but further studies are required before afimoxifene can be approved for this indication and marketed.
Breast cancer classification divides breast cancer into categories according to different schemes criteria and serving a different purpose. The major categories are the histopathological type, the grade of the tumor, the stage of the tumor, and the expression of proteins and genes. As knowledge of cancer cell biology develops these classifications are updated.

Trioxifene, or as the salt trioxifene mesylate (USAN), is a selective estrogen receptor modulator (SERM) with competitive binding activity against estradiol for the ERα and antagonistic activity against ERα-mediated gene expression, that was under preclinical and clinical development by Eli Lilly and Company for breast cancer and prostate cancer, but was abandoned. Its affinity for the rat estrogen receptor was reported to be 20% relative to estradiol.

Elacestrant, sold under the brand name Orserdu, is an anticancer medication which is used in the treatment of breast cancer. It is taken by mouth.
Estrogen deprivation therapy, also known as endocrine therapy, is a form of hormone therapy that is used in the treatment of breast cancer. Modalities include antiestrogens or estrogen blockers such as selective estrogen receptor modulators (SERMs) like tamoxifen, selective estrogen receptor degraders like fulvestrant, and aromatase inhibitors like anastrozole and ovariectomy.
E-SCREEN is a cell proliferation assay based on the enhanced proliferation of human breast cancer cells (MCF-7) in the presence of estrogen active substances. The E-SCREEN test is a tool to easily and rapidly assess estrogenic activity of suspected xenoestrogens. This bioassay measures estrogen-induced increase of the number of human breast cancer cell, which is biologically equivalent to the increase of mitotic activity in tissues of the genital tract. It was originally developed by Soto et al. and was included in the first version of the OECD Conceptual Framework for Testing and Assessment of Endocrine Disrupters published in 2012. However, due to failed validation, it was not included in the updated version of the framework published in 2018.

Endoxifen, also known as 4-hydroxy-N-desmethyltamoxifen, is a nonsteroidal selective estrogen receptor modulator (SERM) of the triphenylethylene group as well as a protein kinase C (PKC) inhibitor. It is under development for the treatment of estrogen receptor-positive breast cancer and for the treatment of mania in bipolar disorder. It is taken by mouth.
Tesmilifene, also known as N,N-diethyl-2-(4-phenylmethyl)ethanamine (DPPE), is a small-molecule antineoplastic drug and chemopotentiator that was under development by YM BioSciences for the treatment of breast cancer in the 2000s but was never marketed. It reached phase III clinical trials for advanced/metastatic breast cancer before development was discontinued.
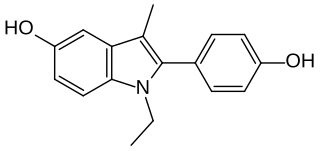
D-15414 is a nonsteroidal weak estrogen of the 2-phenylindole group which was never marketed. It is the major metabolite of the selective estrogen receptor modulator (SERM) zindoxifene (D-16726). D-15414 has high affinity for the estrogen receptor (ER) and inhibits the growth of ER-positive MCF-7 breast cancer cells in vitro. However, contradictorily, subsequent research found that the drug produced fully estrogenic effects in vitro similarly to but less actively than estradiol, with no antiestrogenic activity observed. The reason for the discrepancy between the findings is unclear, though may be due to methodology. The unexpected estrogenic activity of D-15414 may be responsible for the failure of zindoxifene in clinical trials as a treatment for breast cancer.
Benita S. Katzenellenbogen née Schulman is an American physiologist and cell biologist at the University of Illinois at Urbana-Champaign. She has studied cancer, endocrinology, and women's health, focusing on nuclear receptors. She also dedicated efforts to focusing on improving the effectiveness of endocrine therapies in breast cancer.

ERX-11, also known as ERα coregulator-binding modulator-11, is a novel antiestrogen and experimental hormonal antineoplastic agent which is being researched for the potential treatment of estrogen receptor-positive breast cancer. It is not a competitive antagonist of the estrogen receptor (ER) like conventional antiestrogens such as tamoxifen or fulvestrant; instead of binding to the ligand-binding site of the ER, ERX-11 interacts with a different part of the ERα and blocks protein–protein interactions of the ERα with coregulators that are necessary for the receptor to act and regulate gene expression. It was designed to bind to the coregulator binding region of the ERα and inhibit the ERα/coactivator interaction, although its precise binding site and mode of action have yet to be fully elucidated and understood. Nonetheless, it is clear that ERX-11 binds within the AF-2 domain of the ERα.
Endocrine therapy is a common treatment for estrogen receptor positive breast cancer. However, resistance to this therapy can develop, leading to relapse and progression of disease. This highlights the need for new strategies to combat this resistance.













