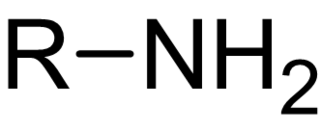
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Formally, amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Polyurethane refers to a class of polymers composed of organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term does not refer to the single type of polymer but a group of polymers. Unlike polyethylene and polystyrene polyurethanes can be produced from a wide range of starting materials resulting various polymers within the same group. This chemical variety produces polyurethanes with different chemical structures leading to many different applications. These include rigid and flexible foams, and coatings, adhesives, electrical potting compounds, and fibers such as spandex and polyurethane laminate (PUL). Foams are the largest application accounting for 67% of all polyurethane produced in 2016.

Petrochemicals are the chemical products obtained from petroleum by refining. Some chemical compounds made from petroleum are also obtained from other fossil fuels, such as coal or natural gas, or renewable sources such as maize, palm fruit or sugar cane.

Epoxy is the family of basic components or cured end products of epoxy resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide functional group is also collectively called epoxy. The IUPAC name for an epoxide group is an oxirane.

Aniline is an organic compound with the formula C6H5NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans.

Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst.

Ethanolamine is a naturally occurring organic chemical compound with the formula HOCH
2CH
2NH
2 or C
2H
7NO. The molecule is bifunctional, containing both a primary amine and a primary alcohol. Ethanolamine is a colorless, viscous liquid with an odor reminiscent of ammonia.

N-Methylethanolamine is an alkanolamine with the formula CH3NHCH2CH2OH. It is flammable, corrosive, colorless, viscous liquid. It is an intermediate in the biosynthesis of choline.
The chemical compound 1,2-dichloroethane, commonly known as ethylene dichloride (EDC), is a chlorinated hydrocarbon. It is a colourless liquid with a chloroform-like odour. The most common use of 1,2-dichloroethane is in the production of vinyl chloride, which is used to make polyvinyl chloride (PVC) pipes, furniture and automobile upholstery, wall coverings, housewares, and automobile parts. 1,2-Dichloroethane is also used generally as an intermediate for other organic chemical compounds, and as a solvent. It forms azeotropes with many other solvents, including water and other chlorocarbons.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.

Diethylenetriamine (abbreviated Dien or DETA) and also known as 2,2’-Iminodi(ethylamine)) is an organic compound with the formula HN(CH2CH2NH2)2. This colourless hygroscopic liquid is soluble in water and polar organic solvents, but not simple hydrocarbons. Diethylenetriamine is structural analogue of diethylene glycol. Its chemical properties resemble those for ethylene diamine, and it has similar uses. It is a weak base and its aqueous solution is alkaline. DETA is a byproduct of the production of ethylenediamine from ethylene dichloride.
Triethylenetetramine (TETA and trien), also known as trientine (INN) when used medically, is an organic compound with the formula [CH2NHCH2CH2NH2]2. The pure freebase is a colorless oily liquid, but, like many amines, older samples assume a yellowish color due to impurities resulting from air-oxidation. It is soluble in polar solvents. The branched isomer tris(2-aminoethyl)amine and piperazine derivatives may also be present in commercial samples of TETA. The hydrochloride salts are used medically as a treatment for copper toxicity.

Diethanolamine, often abbreviated as DEA or DEOA, is an organic compound with the formula HN(CH2CH2OH)2. Pure diethanolamine is a white solid at room temperature, but its tendencies to absorb water and to supercool often results in it being found in a colorless, viscous liquid state. Diethanolamine is polyfunctional, being a secondary amine and a diol. Like other organic amines, diethanolamine acts as a weak base. Reflecting the hydrophilic character of the secondary amine and hydroxyl groups, DEA is soluble in water. Amides prepared from DEA are often also hydrophilic. In 2013, the chemical was classified by the International Agency for Research on Cancer as "possibly carcinogenic to humans" (Group 2B).
Chemical fouling inhibitors are products that are mixtures of fouling and corrosion inhibitors use in boiler feedwater treatment. Several of these products use aliphatic polyamines to coat the surface of pipes.
In chemistry, aminolysis (/am·i·nol·y·sis/) is any chemical reaction in which a molecule is lysed by reacting with ammonia or an amine. The case where the reaction involves ammonia may be more specifically referred to as ammonolysis.

The Wenker synthesis is an organic reaction converting a beta amino alcohol to an aziridine with the help of sulfuric acid. It is used industrially for the synthesis of aziridine itself.
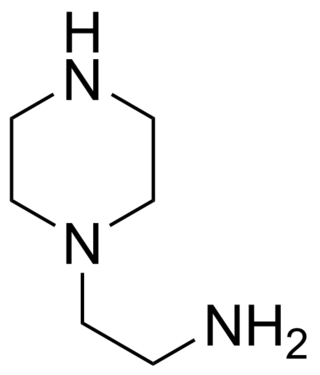
Aminoethylpiperazine (AEP) is a derivative of piperazine. This ethyleneamine contains three nitrogen atoms; one primary, one secondary and one tertiary. It is a corrosive organic liquid and can cause second or third degree burns. Aminoethylpiperazine can also cause pulmonary edema as a result of inhalation. It is REACH and TSCA registered.

Methyldiethanolamine, also known as N-methyl diethanolamine and more commonly as MDEA, is the organic compound with the formula CH3N(C2H4OH)2. It is a colorless liquid with an ammonia odor. It is miscible with water, ethanol and benzene. A tertiary amine, it is widely used as a sweetening agent in chemical, oil refinery, syngas production and natural gas.
A polyamine is an organic compound having more than two amino groups. Alkyl polyamines occur naturally, but some are synthetic. Alkylpolyamines are colorless, hygroscopic, and water soluble. Near neutral pH, they exist as the ammonium derivatives. Most aromatic polyamines are crystalline solids at room temperature.
Tetraethylenepentamine (TEPA) is an organic compound and is in the class of chemicals known as ethyleneamines. It is a slightly viscous liquid and is not colorless but, like many amines, has a yellow color. It is soluble in most polar solvents. Diethylenetriamine (DETA), triethylenetetramine (TETA), piperazine, and aminoethylpiperazine are also usually present in commercial available TEPA.

















