Related Research Articles

Coagulation, also known as clotting, is the process by which blood changes from a liquid to a gel, forming a blood clot. It potentially results in hemostasis, the cessation of blood loss from a damaged vessel, followed by repair. The mechanism of coagulation involves activation, adhesion and aggregation of platelets, as well as deposition and maturation of fibrin.

Thrombin is a serine protease, an enzyme that, in humans, is encoded by the F2 gene. Prothrombin is proteolytically cleaved to form thrombin in the clotting process. Thrombin in turn acts as a serine protease that converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions.

Antithrombin (AT) is a small glycoprotein that inactivates several enzymes of the coagulation system. It is a 464-amino-acid protein produced by the liver. It contains three disulfide bonds and a total of four possible glycosylation sites. α-Antithrombin is the dominant form of antithrombin found in blood plasma and has an oligosaccharide occupying each of its four glycosylation sites. A single glycosylation site remains consistently un-occupied in the minor form of antithrombin, β-antithrombin. Its activity is increased manyfold by the anticoagulant drug heparin, which enhances the binding of antithrombin to factor IIa (thrombin) and factor Xa.

Urokinase, also known as urokinase-type plasminogen activator (uPA), is a serine protease present in humans and other animals. The human urokinase protein was discovered, but not named, by McFarlane and Pilling in 1947. Urokinase was originally isolated from human urine, and it is also present in the blood and in the extracellular matrix of many tissues. The primary physiological substrate of this enzyme is plasminogen, which is an inactive form (zymogen) of the serine protease plasmin. Activation of plasmin triggers a proteolytic cascade that, depending on the physiological environment, participates in thrombolysis or extracellular matrix degradation. This cascade had been involved in vascular diseases and cancer progression.
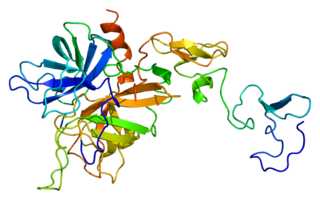
Protein C, also known as autoprothrombin IIA and blood coagulation factor XIX, is a zymogen, that is, an inactive enzyme. The activated form plays an important role in regulating anticoagulation, inflammation, and cell death and maintaining the permeability of blood vessel walls in humans and other animals. Activated protein C (APC) performs these operations primarily by proteolytically inactivating proteins Factor Va and Factor VIIIa. APC is classified as a serine protease since it contains a residue of serine in its active site. In humans, protein C is encoded by the PROC gene, which is found on chromosome 2.

Factor X, also known by the eponym Stuart–Prower factor, is an enzyme of the coagulation cascade. It is a serine endopeptidase. Factor X is synthesized in the liver and requires vitamin K for its synthesis.
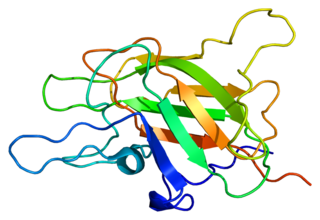
Factor V is a protein of the coagulation system, rarely referred to as proaccelerin or labile factor. In contrast to most other coagulation factors, it is not enzymatically active but functions as a cofactor. Deficiency leads to predisposition for hemorrhage, while some mutations predispose for thrombosis.

Factor XI or plasma thromboplastin antecedent is the zymogen form of factor XIa, one of the enzymes of the coagulation cascade. Like many other coagulation factors, it is a serine protease. In humans, Factor XI is encoded by the F11 gene.
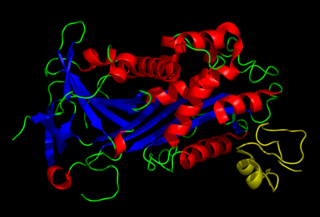
Plasminogen activator inhibitor-1 (PAI-1) also known as endothelial plasminogen activator inhibitor is a protein that in humans is encoded by the SERPINE1 gene. Elevated PAI-1 is a risk factor for thrombosis and atherosclerosis.
Protease-activated receptors(PAR) are a subfamily of related G protein-coupled receptors that are activated by cleavage of part of their extracellular domain. They are highly expressed in platelets, and also on endothelial cells, fibroblasts, immune cells, myocytes, neurons, and tissues that line the gastrointestinal tract.

Hirudin is a naturally occurring peptide in the salivary glands of blood-sucking leeches that has a blood anticoagulant property. This is fundamental for the leeches’ habit of feeding on blood, since it keeps a host's blood flowing after the worm's initial puncture of the skin.

Plasminogen activators are serine proteases that catalyze the activation of plasmin via proteolytic cleavage of its zymogen form plasminogen. Plasmin is an important factor in fibrinolysis, the breakdown of fibrin polymers formed during blood clotting. There are two main plasminogen activators: urokinase (uPA) and tissue plasminogen activator (tPA). Tissue plasminogen activators are used to treat medical conditions related to blood clotting including embolic or thrombotic stroke, myocardial infarction, and pulmonary embolism.
The prothrombinase complex consists of the serine protease, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions. The prothrombinase complex catalyzes the conversion of prothrombin (Factor II), an inactive zymogen, to thrombin (Factor IIa), an active serine protease. The activation of thrombin is a critical reaction in the coagulation cascade, which functions to regulate hemostasis in the body. To produce thrombin, the prothrombinase complex cleaves two peptide bonds in prothrombin, one after Arg271 and the other after Arg320. Although it has been shown that Factor Xa can activate prothrombin when unassociated with the prothrombinase complex, the rate of thrombin formation is severely decreased under such circumstances. The prothrombinase complex can catalyze the activation of prothrombin at a rate 3 x 105-fold faster than can Factor Xa alone. Thus, the prothrombinase complex is required for the efficient production of activated thrombin and also for adequate hemostasis.

Thrombomodulin (TM), CD141 or BDCA-3 is an integral membrane protein expressed on the surface of endothelial cells and serves as a cofactor for thrombin. It reduces blood coagulation by converting thrombin to an anticoagulant enzyme from a procoagulant enzyme. Thrombomodulin is also expressed on human mesothelial cell, monocyte and a dendritic cell subset.
Kininogens are precursor proteins for kinins, biologically active polypeptides involved in blood coagulation, vasodilation, smooth muscle contraction, inflammatory regulation, and the regulation of the cardiovascular and renal systems.
Direct thrombin inhibitors (DTIs) are a class of medication that act as anticoagulants by directly inhibiting the enzyme thrombin. Some are in clinical use, while others are undergoing clinical development. Several members of the class are expected to replace heparin and warfarin in various clinical scenarios.

Protein C inhibitor is a serine protease inhibitor (serpin) that limits the activity of protein C.
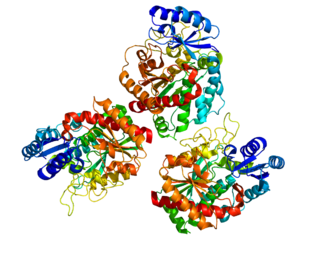
Carboxypeptidase B2 (CPB2), also known as carboxypeptidase U (CPU), plasma carboxypeptidase B (pCPB) or thrombin-activatable fibrinolysis inhibitor (TAFI), is an enzyme that, in humans, is encoded by the gene CPB2.
Direct thrombin inhibitors (DTIs) are a class of anticoagulant drugs that can be used to prevent and treat embolisms and blood clots caused by various diseases. They inhibit thrombin, a serine protease which affects the coagulation cascade in many ways. DTIs have undergone rapid development since the 90's. With technological advances in genetic engineering the production of recombinant hirudin was made possible which opened the door to this new group of drugs. Before the use of DTIs the therapy and prophylaxis for anticoagulation had stayed the same for over 50 years with the use of heparin derivatives and warfarin which have some well known disadvantages. DTIs are still under development, but the research focus has shifted towards factor Xa inhibitors, or even dual thrombin and fXa inhibitors that have a broader mechanism of action by both inhibiting factor IIa (thrombin) and Xa. A recent review of patents and literature on thrombin inhibitors has demonstrated that the development of allosteric and multi-mechanism inhibitors might lead the way to a safer anticoagulant.
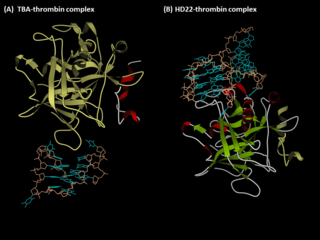
Anti-thrombin aptamers are G-quadruplex-bearing oligonucleotides, which recognizes the exosites of human thrombin. The first anti-thrombin aptamer, TBA, was generated through via SELEX technology in 1992 by L.C. Bock, J.J. Toole and colleagues. A second thrombin-binding aptamer, HD22, recognizes thrombin exosite II and was discovered in 1997 by NeXstar. These two aptamers have high affinity and good specificity and have been widely studied and used for the development of aptamer-based therapeutics and diagnostics.
References
- ↑ Yegneswaran, S.; Tiefenbrunn, T. K.; Fernández, J. A.; Dawson, P. E. (October 2007). "Manipulation of thrombin exosite I, by ligand-directed covalent modification". Journal of Thrombosis and Haemostasis. 5 (10): 2062–2069. doi:10.1111/j.1538-7836.2007.02712.x. PMID 17883702. S2CID 38198958.
- ↑ Lockett, J. M.; Sheehan, J. P.; Mast, A. E. (July 2003). "Binding at two distinct exosites is required for activation of prothrombin by prothrombinase". Journal of Thrombosis and Haemostasis. 1 (Supplement 1). Archived from the original on 2004-11-26.
- ↑ Müller, Jens; Isermann, Berend; Dücker, Christina; Salehi, Mohammad; Meyer, Moritz; Friedrich, Max; Madhusudhan, Thati; Oldenburg, Johannes; Mayer, Günter; Pötzsch, Bernd (April 2009). "An Exosite-Specific ssDNA Aptamer Inhibits the Anticoagulant Functions of Activated Protein C and Enhances Inhibition by Protein C Inhibitor". Chemistry & Biology. 16 (4): 442–451. doi:10.1016/j.chembiol.2009.03.007. PMID 19389630.
- ↑ Serine Endopeptidases: Advances in Research and Application: 2011 Edition. ScholarlyEditions. 2012. ISBN 978-1-4649-2658-7.[ page needed ]