A glycosidic bond or glycosidic linkage is a type of covalent bond that joins a carbohydrate (sugar) molecule to another group, which may or may not be another carbohydrate.
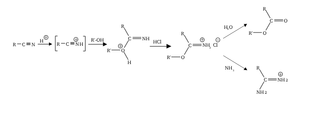
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt ; this is sometimes referred to as a Pinner salt. The reaction is named after Adolf Pinner, who first described it in 1877. Pinner salts are themselves reactive and undergo additional nucleophilic additions to give various useful products:
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today antimigraine drugs of the triptan class are often synthesized by this method.
Carl Ulrich Franz Mannich was a German chemist. From 1927 to 1943 he was professor for pharmaceutical chemistry at the University of Berlin. His areas of expertise were keto bases, alcohol bases, derivatives of piperidine, papaverine, lactones and also Digitalis-glycosides.
The Japp–Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids and aryl diazonium salts. The reaction is named after the chemists Francis Robert Japp and Felix Klingemann.
The Bischler–Möhlau indole synthesis, also often referred to as "The Bischler Indole Synthesis," is a chemical reaction that forms a 2-aryl-indole from an α-bromo-acetophenone and excess aniline; it is named after August Bischler and Richard Möhlau .

The Reimer–Tiemann reaction is a chemical reaction used for the ortho-formylation of phenols; with the simplest example being the conversion of phenol to salicylaldehyde. The reaction was discovered by Karl Reimer and Ferdinand Tiemann. The Reimer in question was Karl Reimer (1845-1883) not the lesser known Carl Ludwig Reimer (1856-1921).

Phoebus Aaron Theodore Levene was a Russian born American biochemist who studied the structure and function of nucleic acids. He characterized the different forms of nucleic acid, DNA from RNA, and found that DNA contained adenine, guanine, thymine, cytosine, deoxyribose, and a phosphate group.

Hans von Pechmann was a German chemist, renowned for his discovery of diazomethane in 1894. Pechmann condensation and Pechmann pyrazole synthesis. He also first prepared 1,2-diketones, acetonedicarboxylic acid, methylglyoxal and diphenyltriketone; established the symmetrical structure of anthraquinone.
The Koenigs–Knorr reaction in organic chemistry is the substitution reaction of a glycosyl halide with an alcohol to give a glycoside. It is one of the oldest glycosylation reactions. It is named after Wilhelm Koenigs (1851–1906), a student of von Bayer and fellow student with Hermann Emil Fischer, and Edward Knorr, a student of Koenigs.

Osazones are a class of carbohydrate derivatives found in organic chemistry formed when reducing sugars are reacted with excess of phenylhydrazine at boiling temperatures.
The Schotten–Baumann reaction is a method to synthesize amides from amines and acid chlorides:

Johann Peter Griess was an industrial chemist and an early pioneer of organic chemistry.

Oskar Piloty was a German chemist.

Hans Heinrich Landolt was a Swiss chemist who discovered iodine clock reaction. He is also one of the founders of Landolt–Börnstein database. He tested law of mass conservation which was given by Lavoisier.
A chemical glycosylation reaction involves the coupling of a glycosyl donor, to a glycosyl acceptor forming a glycoside. If both the donor and acceptor are sugars, then the product is an oligosaccharide. The reaction requires activation with a suitable activating reagent. The reactions often result in a mixture of products due to the creation of a new stereogenic centre at the anomeric position of the glycosyl donor. The formation of a glycosidic linkage allows for the synthesis of complex polysaccharides which may play important roles in biological processes and pathogenesis and therefore having synthetic analogs of these molecules allows for further studies with respect to their biological importance.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.

Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide. As a bifunctional compound, trichloroacetonitrile can react at both the trichloromethyl and the nitrile group. The electron-withdrawing effect of the trichloromethyl group activates the nitrile group for nucleophilic additions. The high reactivity makes trichloroacetonitrile a versatile reagent, but also causes its susceptibility towards hydrolysis.

Géza Gusztáv Zemplén, Ph.D. was a notable Hungarian chemist, organic chemist, professor, and chemistry author. He was a recipient of the Kossuth Prize, a member of the Hungarian Academy of Sciences, and was the brother of Professor Győző Zemplén. His major field of research was structural chemistry and biochemistry including the synthesis of naturally occurring flavonoid-glycosides.













