Related Research Articles

A nickel–metal hydride battery is a type of rechargeable battery. The chemical reaction at the positive electrode is similar to that of the nickel-cadmium cell (NiCd), with both using nickel oxide hydroxide (NiOOH). However, the negative electrodes use a hydrogen-absorbing alloy instead of cadmium. NiMH batteries can have two to three times the capacity of NiCd batteries of the same size, with significantly higher energy density, although only about half that of lithium-ion batteries.

The nickel–cadmium battery is a type of rechargeable battery using nickel oxide hydroxide and metallic cadmium as electrodes. The abbreviation Ni–Cd is derived from the chemical symbols of nickel (Ni) and cadmium (Cd): the abbreviation NiCad is a registered trademark of SAFT Corporation, although this brand name is commonly used to describe all Ni–Cd batteries.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.

A rechargeable battery, storage battery, or secondary cell, is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer.

A lithium polymer battery, or more correctly lithium-ion polymer battery, is a rechargeable battery of lithium-ion technology using a polymer electrolyte instead of a liquid electrolyte. Highly conductive semisolid (gel) polymers form this electrolyte. These batteries provide higher specific energy than other lithium battery types and are used in applications where weight is a critical feature, such as mobile devices, radio-controlled aircraft and some electric vehicles.
Memory effect, also known as battery effect, lazy battery effect, or battery memory, is an effect observed in nickel-cadmium rechargeable batteries that causes them to hold less charge. It describes the situation in which nickel-cadmium batteries gradually lose their maximum energy capacity if they are repeatedly recharged after being only partially discharged. The battery appears to "remember" the smaller capacity.

An automotive battery, or car battery, is a rechargeable battery that is used to start a motor vehicle. Its main purpose is to provide an electric current to the electric-powered starting motor, which in turn starts the chemically-powered internal combustion engine that actually propels the vehicle. Once the engine is running, power for the car's electrical systems is still supplied by the battery, with the alternator charging the battery as demands increase or decrease.

The AA battery is a standard size single cell cylindrical dry battery. The IEC 60086 system calls the size R6, and ANSI C18 calls it 15. It is named UM-3 by JIS of Japan. Historically, it is known as D14, U12 – later U7, or HP7 in official documentation in the United Kingdom, or a pen cell.

A battery charger, recharger, or simply charger, is a device that stores energy in a battery by running an electric current through it. The charging protocol depends on the size and type of the battery being charged. Some battery types have high tolerance for overcharging and can be recharged by connection to a constant voltage source or a constant current source, depending on battery type. Simple chargers of this type must be manually disconnected at the end of the charge cycle. Other battery types use a timer to cut off when charging should be complete. Other battery types cannot withstand over-charging, becoming damaged, over heating or even exploding. The charger may have temperature or voltage sensing circuits and a microprocessor controller to safely adjust the charging current and voltage, determine the state of charge, and cut off at the end of charge. Chargers may elevate the output voltage proportionally with current to compensate for impedance in the wires.

A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes.

The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode. Because of their lower cost, high safety, low toxicity, long cycle life and other factors, LFP batteries are finding a number of roles in vehicle use, utility-scale stationary applications, and backup power. LFP batteries are cobalt-free. As of September 2022, LFP type battery market share for EVs reached 31%, and of that, 68% was from Tesla and Chinese EV maker BYD production alone. Chinese manufacturers currently hold a near monopoly of LFP battery type production. With patents having started to expire in 2022 and the increased demand for cheaper EV batteries, LFP type production is expected to rise further and surpass lithium nickel manganese cobalt oxides (NMC) type batteries in 2028.

Lithium iron phosphate or lithium ferro-phosphate (LFP) is an inorganic compound with the formula LiFePO
4. It is a gray, red-grey, brown or black solid that is insoluble in water. The material has attracted attention as a component of lithium iron phosphate batteries, a type of Li-ion battery. This battery chemistry is targeted for use in power tools, electric vehicles, solar energy installations and more recently large grid-scale energy storage.
The lithium-titanate or lithium-titanium-oxide (LTO) battery is a type of rechargeable battery which has the advantage of being faster to charge than other lithium-ion batteries but the disadvantage of having a much lower energy density.
A battery management system (BMS) is any electronic system that manages a rechargeable battery, such as by protecting the battery from operating outside its safe operating area, monitoring its state, calculating secondary data, reporting that data, controlling its environment, authenticating it and / or balancing it.

A battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons that will flow through an external electric circuit to the positive terminal. When a battery is connected to an external electric load, a redox reaction converts high-energy reactants to lower-energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell.

IUoU is a DIN-designation for a lead-acid battery charging procedure that is also known as 3-stage charging, 3-phase charging, or 3-step charging. It consists of three phases, to be executed by a battery charger. The three phases are: I-phase, Uo-phase, and U-phase. The purpose is to fully charge the battery in a relatively short time without reducing its life span and to keep the battery charged indefinitely as long as the charger is connected.

Battery balancing and battery redistribution refer to techniques that improve the available capacity of a battery pack with multiple cells and increase each cell's longevity. A battery balancer or battery regulator is an electrical device in a battery pack that performs battery balancing. Balancers are often found in lithium-ion battery packs for laptop computers, electrical vehicles. etc.

Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as its charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the cathode material. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. In other cases (such as aqueous Na-ion batteries) they are quite different from Li-ion batteries.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.
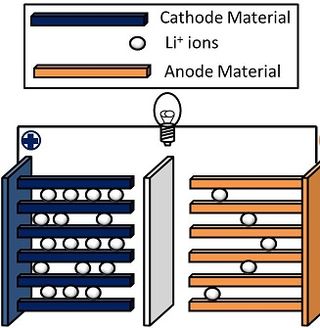
Lithium nickel manganese cobalt oxides (abbreviated NMC, Li-NMC, LNMC, or NCM) are mixed metal oxides of lithium, nickel, manganese and cobalt with the general formula LiNixMnyCo1-x-yO2. These materials are commonly used in lithium-ion batteries for mobile devices and electric vehicles, acting as the positively charged cathode.
References
- ↑ Team, M.I.T. Electric Vehicle, A Guide to Understanding Battery Specifications (PDF), retrieved 2012-01-12
- ↑ Bill Moeller; Jan Moeller (1 October 1994). RV Electrical Systems: A Basic Guide to Troubleshooting, Repairing and Improvement. McGraw-Hill Professional. p. 34. ISBN 978-0-07-042778-5 . Retrieved 12 January 2012.
- ↑ Whitham D. Reeve (2007). DC power system design for telecommunications. John Wiley and Sons. p. 239. ISBN 978-0-471-68161-8 . Retrieved 12 January 2012.
- ↑ "Float charging lithium ion cells". Electronics Weekly.com. February 2006. Retrieved 4 September 2018.
- ↑ John A. O'Connor, Unitrode Application Note: Simple Switchmode Lead-Acid Battery Charger (PDF), retrieved 2012-11-10