Related Research Articles

A capsid is the protein shell of a virus, enclosing its genetic material. It consists of several oligomeric (repeating) structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The proteins making up the capsid are called capsid proteins or viral coat proteins (VCP). The capsid and inner genome is called the nucleocapsid.

In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding of large proteins or macromolecular protein complexes. There are a number of classes of molecular chaperones, all of which function to assist large proteins in proper protein folding during or after synthesis, and after partial denaturation. Chaperones are also involved in the translocation of proteins for proteolysis.
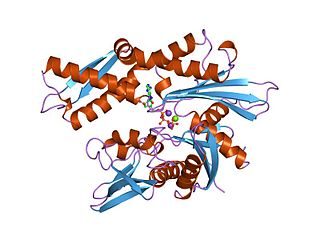
The 70 kilodalton heat shock proteins are a family of conserved ubiquitously expressed heat shock proteins. Proteins with similar structure exist in virtually all living organisms. Intracellularly localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from the adverse effects of physiological stresses. Additionally, membrane-bound Hsp70s have been identified as a potential target for cancer therapies and their extracellularly localized counterparts have been identified as having both membrane-bound and membrane-free structures.
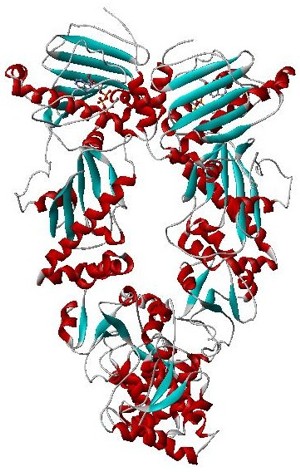
Hsp90 is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation. It also stabilizes a number of proteins required for tumor growth, which is why Hsp90 inhibitors are investigated as anti-cancer drugs.

GroEL is a protein which belongs to the chaperonin family of molecular chaperones, and is found in many bacteria. It is required for the proper folding of many proteins. To function properly, GroEL requires the lid-like cochaperonin protein complex GroES. In eukaryotes the organellar proteins Hsp60 and Hsp10 are structurally and functionally nearly identical to GroEL and GroES, respectively, due to their endosymbiotic origin.
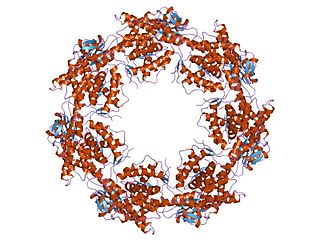
HSP60, also known as chaperonins (Cpn), is a family of heat shock proteins originally sorted by their 60kDa molecular mass. They prevent misfolding of proteins during stressful situations such as high heat, by assisting protein folding. HSP60 belong to a large class of molecules that assist protein folding, called molecular chaperones.

The Max Planck Institute of Biochemistry (MPIB) is a research institute of the Max Planck Society located in Martinsried, a suburb of Munich. The institute was founded in 1973 by the merger of three formerly independent institutes: the Max Planck Institute of Biochemistry, the Max Planck Institute of Protein and Leather Research, and the Max Planck Institute of Cell Chemistry.
Co-chaperones are proteins that assist chaperones in protein folding and other functions. Co-chaperones are the non-client binding molecules that assist in protein folding mediated by Hsp70 and Hsp90. They are particularly essential in stimulation of the ATPase activity of these chaperone proteins. There are a great number of different co-chaperones however based on their domain structure most of them fall into two groups: J-domain proteins and tetratricopeptide repeats (TPR).

Heat shock protein HSP 90-beta also called HSP90beta is a protein that in humans is encoded by the HSP90AB1 gene.

DnaJ homolog subfamily A member 3, mitochondrial, also known as Tumorous imaginal disc 1 (TID1), is a protein that in humans is encoded by the DNAJA3 gene on chromosome 16. This protein belongs to the DNAJ/Hsp40 protein family, which is known for binding and activating Hsp70 chaperone proteins to perform protein folding, degradation, and complex assembly. As a mitochondrial protein, it is involved in maintaining membrane potential and mitochondrial DNA (mtDNA) integrity, as well as cellular processes such as cell movement, growth, and death. Furthermore, it is associated with a broad range of diseases, including neurodegenerative diseases, inflammatory diseases, and cancers.

Binding immunoglobulin protein (BiPS) also known as 78 kDa glucose-regulated protein (GRP-78) or heat shock 70 kDa protein 5 (HSPA5) is a protein that in humans is encoded by the HSPA5 gene.

Heat shock 70 kDa protein 4 is a protein that in humans is encoded by the HSPA4 gene.

DnaJ homolog subfamily B member 1 is a protein that in humans is encoded by the DNAJB1 gene.

DnaJ homolog subfamily B member 6 is a protein that in humans is encoded by the DNAJB6 gene.

Histone chaperone ASF1B is a protein that in humans is encoded by the ASF1B gene.

In molecular biology, chaperone DnaJ, also known as Hsp40, is a molecular chaperone protein. It is expressed in a wide variety of organisms from bacteria to humans.

G. Marius Clore MAE, FRSC, FRS is a British-born, Anglo-American molecular biophysicist and structural biologist. He was born in London, U.K. and is a dual U.S./U.K. Citizen. He is a Member of the National Academy of Sciences, a Fellow of the Royal Society, a NIH Distinguished Investigator, and the Chief of the Molecular and Structural Biophysics Section in the Laboratory of Chemical Physics of the National Institute of Diabetes and Digestive and Kidney Diseases at the U.S. National Institutes of Health. He is known for his foundational work in three-dimensional protein and nucleic acid structure determination by biomolecular NMR spectroscopy, for advancing experimental approaches to the study of large macromolecules and their complexes by NMR, and for developing NMR-based methods to study rare conformational states in protein-nucleic acid and protein-protein recognition. Clore's discovery of previously undetectable, functionally significant, rare transient states of macromolecules has yielded fundamental new insights into the mechanisms of important biological processes, and in particular the significance of weak interactions and the mechanisms whereby the opposing constraints of speed and specificity are optimized. Further, Clore's work opens up a new era of pharmacology and drug design as it is now possible to target structures and conformations that have been heretofore unseen.

Chromatin assembly factor-1 (CAF-1) is a protein complex — including Chaf1a (p150), Chaf1b (p60), and p48 subunits in humans, or Cac1, Cac2, and Cac3, respectively, in yeast— that assembles histone tetramers onto replicating DNA during the S phase of the cell cycle.

Sue Hengren Wickner is an American biochemist and geneticist who is a distinguished investigator and the head of the DNA Molecular Biology section of the National Institutes of Health. Her laboratory is under the National Cancer Institute and is located in the Center for Cancer Research (NCI/CCR).

GrpE is a bacterial nucleotide exchange factor that is important for regulation of protein folding machinery, as well as the heat shock response. It is a heat-inducible protein and during stress it prevents unfolded proteins from accumulating in the cytoplasm. Accumulation of unfolded proteins in the cytoplasm can lead to cell death.
References
- ↑ Hoffmann, J. R. H.; Linke, K.; Graf, P. C.; Lilie, H.; Jakob, U. (2003). "Identification of a redox-regulated chaperone network". The EMBO Journal. 23 (1): 160–168. doi:10.1038/sj.emboj.7600016. PMC 1271656 . PMID 14685279.