Mechanochemistry is the initiation of chemical reactions by mechanical phenomena. Mechanochemistry thus represents a fourth way to cause chemical reactions, complementing thermal reactions in fluids, photochemistry, and electrochemistry. Conventionally mechanochemistry focuses on the transformations of covalent bonds by mechanical force. Not covered by the topic are many phenomena: phase transitions, dynamics of biomolecules, and sonochemistry.

Pentalene is a polycyclic hydrocarbon composed of two fused cyclopentadiene rings. It has chemical formula C8H6. It is antiaromatic, because it has 4n π electrons where n is any integer. For this reason it dimerizes even at temperatures as low as −100 °C. The derivative 1,3,5-tri-tert-butylpentalene was synthesized in 1973. Because of the tert-butyl substituents this compound is thermally stable. Pentalenes can also be stabilized by benzannulation for example in the compounds benzopentalene and dibenzopentalene.
In organic chemistry, arynes and benzynes are a class of highly reactive chemical species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of alkynes under high strain.

In organic chemistry, hyperconjugation refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. However, sometimes, low-lying antibonding σ* orbitals may also interact with filled orbitals of lone pair character (n) in what is termed negative hyperconjugation. Increased electron delocalization associated with hyperconjugation increases the stability of the system. In particular, the new orbital with bonding character is stabilized, resulting in an overall stabilization of the molecule. Only electrons in bonds that are in the β position can have this sort of direct stabilizing effect — donating from a sigma bond on an atom to an orbital in another atom directly attached to it. However, extended versions of hyperconjugation can be important as well. The Baker–Nathan effect, sometimes used synonymously for hyperconjugation, is a specific application of it to certain chemical reactions or types of structures.

A persistent carbene is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet, but does not exhibit the tremendous instability typically associated with such moieties. The best-known examples and by far largest subgroup are the N-heterocyclic carbenes (NHC), in which nitrogen atoms flank the formal carbene.
In organic chemistry, a cycloalkyne is the cyclic analog of an alkyne. A cycloalkyne consists of a closed ring of carbon atoms containing one or more triple bonds. Cycloalkynes have a general formula CnH2n−4. Because of the linear nature of the C−C≡C−C alkyne unit, cycloalkynes can be highly strained and can only exist when the number of carbon atoms in the ring is great enough to provide the flexibility necessary to accommodate this geometry. Large alkyne-containing carbocycles may be virtually unstrained, while the smallest constituents of this class of molecules may experience so much strain that they have yet to be observed experimentally. Cyclooctyne is the smallest cycloalkyne capable of being isolated and stored as a stable compound. Despite this, smaller cycloalkynes can be produced and trapped through reactions with other organic molecules or through complexation to transition metals.
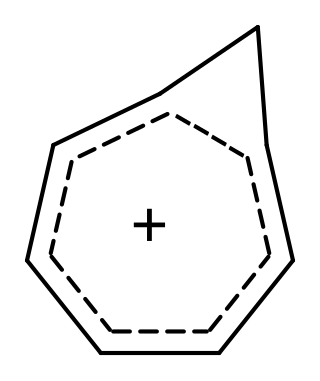
Homoaromaticity, in organic chemistry, refers to a special case of aromaticity in which conjugation is interrupted by a single sp3 hybridized carbon atom. Although this sp3 center disrupts the continuous overlap of p-orbitals, traditionally thought to be a requirement for aromaticity, considerable thermodynamic stability and many of the spectroscopic, magnetic, and chemical properties associated with aromatic compounds are still observed for such compounds. This formal discontinuity is apparently bridged by p-orbital overlap, maintaining a contiguous cycle of π electrons that is responsible for this preserved chemical stability.

Maitotoxin (MTX) is an extremely potent biotoxin produced by Gambierdiscus toxicus, a dinoflagellate species. Maitotoxin has been shown to be more than one hundred thousand times as potent as VX nerve agent. Maitotoxin is so potent that it has been demonstrated that an intraperitoneal injection of 130 ng/kg was lethal in mice. Maitotoxin was named from the ciguateric fish Ctenochaetus striatus—called "maito" in Tahiti—from which maitotoxin was isolated for the first time. It was later shown that maitotoxin is actually produced by the dinoflagellate Gambierdiscus toxicus.
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of intermolecular interactions. It is an interdisciplinary academic field, bridging solid-state and supramolecular chemistry.

In organic chemistry, the acenes or polyacenes are a class of organic compounds and polycyclic aromatic hydrocarbons made up of benzene rings which have been linearly fused. They follow the general molecular formula C4n+2H2n+4.

Cyclooctane is a cycloalkane with the molecular formula (CH2)8. It is a simple colourless hydrocarbon, but it is often a reference compound for saturated eight-membered ring compounds in general.
Thiosulfuric acid is the inorganic compound with the formula H2S2O3. It has attracted academic interest as a simple, easily accessed compound that is labile. It has few practical uses.

Dynamic combinatorial chemistry (DCC); also known as constitutional dynamic chemistry (CDC) is a method to the generation of new molecules formed by reversible reaction of simple building blocks under thermodynamic control. The library of these reversibly interconverting building blocks is called a dynamic combinatorial library (DCL). All constituents in a DCL are in equilibrium, and their distribution is determined by their thermodynamic stability within the DCL. The interconversion of these building blocks may involve covalent or non-covalent interactions. When a DCL is exposed to an external influence, the equilibrium shifts and those components that interact with the external influence are stabilised and amplified, allowing more of the active compound to be formed.
Osmocene is an organoosmium compound found as a white solid. It is a metallocene with the formula Os(C5H5)2.
Ayyappanpillai Ajayaghosh is a research scientist/academician in the domain of interdisciplinary chemistry, and the former Director of the National Institute for Interdisciplinary Science and Technology. He is known for his studies on supramolecular assemblies, organogels, photoresponsive materials, chemosensory and security materials systems and is an elected fellow of all the three major Indian science academies viz. the National Academy of Sciences, India, Indian National Science Academy and the Indian Academy of Sciences as well as The World Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Chemical Sciences in 2007. He is the first chemist to receive the Infosys Science Prize for physical sciences, awarded by the Infosys Science Foundation. He received the TWAS Prize of The World Academy of Sciences in 2013 and the Goyal prize in 2019.

1,6-Methano[10]annulene (also known as 1,6-methanonaphthalene or homonaphthalene) is an aromatic hydrocarbon with chemical formula C11H10. It was the first stable aromatic compound based on the cyclodecapentaene system to be discovered.
Sodium hypobromite is an inorganic compound with the chemical formula NaOBr. It is a sodium salt of hypobromous acid. It consists of sodium cations Na+ and hypobromite anions −OBr. It is usually obtained as the pentahydrate, so the compound that is usually called sodium hypobromite actually has the formula NaOBr·5H2O. It is a yellow-orange solid that is soluble in water. It adopts a monoclinic crystal structure with a Br–O bond length of 1.820 Å. It is the bromine analogue of sodium hypochlorite, the active ingredient in common bleach. In practice the salt is usually encountered as an aqueous solution.

Stephen L. Craig is the William T. Miller Professor of Chemistry at Duke University. He is the director of the Center for Molecularly Optimized Networks, a NSF Center for Chemical Innovation.
Lynda Soderholm is a physical chemist at the U.S. Department of Energy's (DOE) Argonne National Laboratory with a specialty in f-block elements. She is a senior scientist and the lead of the Actinide, Geochemistry & Separation Sciences Theme within Argonne's Chemical Sciences and Engineering Division. Her specific role is the Separation Science group leader within Heavy Element Chemistry and Separation Science (HESS), directing basic research focused on low-energy methods for isolating lanthanide and actinide elements from complex mixtures. She has made fundamental contributions to understanding f-block chemistry and characterizing f-block elements.
Tomislav Friščić holds the Leverhulme International Professorship and Chair in Green and Sustainable chemistry at the University of Birmingham. His research focus is at the interface of green chemistry and materials science, developing solvent-free chemistry and mechanochemistry for the cleaner, efficient synthesis of molecules and materials, including organic solids such as pharmaceutical cocrystals, coordination polymers and Metal-Organic Frameworks (MOFs), and a wide range of organic targets such as active pharmaceutical ingredients. He is a Fellow of the Royal Society of Chemistry (RSC), member of the College of New Scholars, Artists and Scientists of the Royal Society of Canada and a corresponding member of the Croatian Academy of Sciences and Arts. He has served on the Editorial Board of CrystEngComm, the Early Career Board of the ACS journal ACS Sustainable Chemistry & Engineering, and was an Associate Editor for the journal Molecular Crystals & Liquid Crystals as well as for the journal Synthesis. He was a Topic Editor and Social Media Editor, and is currently a member of the Editorial Advisory Board of the journal Crystal Growth & Design published by the American Chemical Society (ACS). He famously has a dog named Zizi.











