Related Research Articles

Ethylene glycol is an organic compound with the formula (CH2OH)2. It is mainly used for two purposes: as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. It has a sweet taste, but is toxic in high concentrations. This molecule has been observed in outer space.

Novartis AG is a Swiss multinational pharmaceutical corporation based in Basel, Switzerland. Consistently ranked in the global top five, Novartis is one of the largest pharmaceutical companies in the world and was the fourth largest by revenue in 2022.
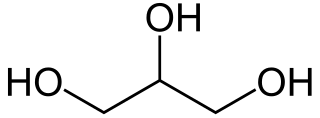
Glycerol is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature.

Cold medicines are a group of medications taken individually or in combination as a treatment for the symptoms of the common cold and similar conditions of the upper respiratory tract. The term encompasses a broad array of drugs, including analgesics, antihistamines and decongestants, among many others. It also includes drugs which are marketed as cough suppressants or antitussives, but their effectiveness in reducing cough symptoms is unclear or minimal.
The Medicines and Healthcare products Regulatory Agency (MHRA) is an executive agency of the Department of Health and Social Care in the United Kingdom which is responsible for ensuring that medicines and medical devices work and are acceptably safe.

Diethylene glycol (DEG) is an organic compound with the formula (HOCH2CH2)2O. It is a colorless, practically odorless, and hygroscopic liquid with a sweetish taste. It is a four carbon dimer of ethylene glycol. It is miscible in water, alcohol, ether, acetone, and ethylene glycol. DEG is a widely used solvent. It can be a normal ingredient in various consumer products, and it can be a contaminant. DEG has also been misused to sweeten wine and beer, and to viscosify oral and topical pharmaceutical products. Its use has resulted in many epidemics of poisoning since the early 20th century.
The pharmaceutical industry in India was valued at an estimated US$42 billion in 2021 and is estimated to reach $130 billion by 2030. India is the world's largest provider of generic medicines by volume, with a 20% share of total global pharmaceutical exports. It is also the largest vaccine supplier in the world by volume, accounting for more than 60% of all vaccines manufactured in the world. Indian pharmaceutical products are exported to various regulated markets including the US, UK, European Union and Canada.
Since the 1990s, several mass poisonings from toxic cough syrup have occurred in developing countries. In these cases, an ingredient in cough syrup, glycerine (glycerol), was replaced with diethylene glycol, a cheaper alternative to glycerine for industrial applications. Diethylene glycol is nephrotoxic and can result in multiple organ dysfunction syndrome (MODS), especially in children.

Zheng Xiaoyu was the director of the State Food and Drug Administration of the People's Republic of China from 2003 to 2005. He was sentenced to death for corruption and allowing possibly tainted products in mainland China in the first instance trial at Beijing No.1 Intermediate Court on May 29, 2007. He was executed on July 10, 2007.
In 2007, a series of product recalls and import bans were imposed by the product safety institutions of the United States, Canada, Western Europe, Australia, and New Zealand against products manufactured in and exported from the mainland of the People's Republic of China (PRC) because of numerous alleged consumer safety issues. The many product recalls within the year led Consumer Reports and other observers to dub 2007 "The Year of the Recall.”
Eduardo Arias was a Panamanian Guna, whose discovery of contaminated toothpaste saved lives by alerting the public to potentially poisonous products purchased from the People's Republic of China (PRC).

The 2008 Chinese milk scandal was a significant food safety incident in China. The scandal involved Sanlu Group's milk and infant formula along with other food materials and components being adulterated with the chemical melamine, which resulted in kidney stones and other kidney damage in infants. The chemical was used to increase the nitrogen content of diluted milk, giving it the appearance of higher protein content in order to pass quality control testing. 300,000 affected children were identified, among which 54,000 were hospitalized, according to the latest report in January 2009. The deaths of six babies were officially concluded to be related to the contaminated milk.
A counterfeit medication or a counterfeit drug is a medication or pharmaceutical item which is produced and sold with the intent to deceptively represent its origin, authenticity, or effectiveness. A counterfeit drug may contain inappropriate quantities of active ingredients, or none, may be improperly processed within the body, may contain ingredients that are not on the label, or may be supplied with inaccurate or fake packaging and labeling.

Wockhardt is an Indian pharmaceutical and biotechnology company headquartered in Mumbai, India. It produces formulations, biopharmaceuticals, nutrition products, vaccines and active pharmaceutical ingredients (APIs). The company has manufacturing plants in India, UK, Ireland, France and US, and subsidiaries in US, UK, Ireland and France.
In February–March 2013 several European countries, including Romania, Serbia, Croatia reported nationwide contamination of milk for human consumption with aflatoxins. The details are still scarce.
Events in the year 2022 in the Gambia.

The Truth Pill: The Myth of Drug Regulation in India is a 2022 book by whistleblower Dinesh Thakur and lawyer Prashant Reddy. The book highlights the problems in India's drug regulatory framework, and the government oversight relating to poor manufacturing practices and clinical trials of drugs by Indian pharmaceutical companies.

The Uzbekistan cough syrup scandal was a series of poisonings that resulted in the deaths of 18 children in Samarkand and two more children elsewhere in Uzbekistan in December 2022 and January 2023. It was caused by the toxic levels of diethylene glycol and ethylene glycol in cold medicines produced by the Indian company Marion Biotech, such as the Dok-1 Max brand. Subsequently, the Indian government investigated Marion Biotech's manufacturing processes, while Uzbek authorities opened a criminal case against members of the health system that had contributed to the children's deaths, such as regulatory officials and pharmacy administrators.
References
- ↑ "Medical Product Alert N°6/2022: Substandard (contaminated) paediatric medicines". www.who.int. World Health Organization.
- ↑ "India-made cough syrups may be tied to 66 deaths in Gambia: WHO". Al Jazeera. 5 October 2022.
- ↑ Das, Krishna N. (7 October 2022). "India tests samples of cough syrup linked to deaths of children in Gambia". Reuters.
- ↑ "Gambia cough syrup scandal: Mothers demand justice". BBC News. 7 October 2022.
- ↑ "Gambia cough syrup scandal: Police investigate deaths linked to Indian medicine". BBC News. 9 October 2022.
- ↑ "Cough-syrup scandal: How did it end up in The Gambia?". BBC News. 12 October 2022.
- ↑ "India finds serious breaches, halts production over Gambia deaths". Al Jazeera. 12 October 2022.
- ↑ "How 'Made in India' cough syrups ended up killing nearly 70 children in Gambia". The Independent. 12 October 2022.
- ↑ "Explained | The Gambia deaths and the toxic cough syrups that are causing them". The Hindu. 17 October 2022.
- ↑ Lawal, Shola (9 November 2022). "Families Whose Kids Died in Cough Syrup Scandal Offered $280 in Compensation". Vice.
- ↑ "As African kids died, doctors fought for ban on toxic Indian syrup". Reuters. 10 March 2023.
- ↑ "Cough Drops" . Retrieved 17 October 2023.
- ↑ "India probes bribery claim in toxic syrup tests". The Economic Times. 13 June 2023.
- ↑ Mcallister, Edward (2023-06-30). "Exclusive: Gambia families sue Indian drugmaker after cough syrup deaths". Reuters. Retrieved 2023-07-04.