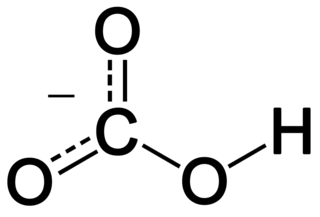
In inorganic chemistry, bicarbonate is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula HCO−
3.

Hypoxia is a condition in which the body or a region of the body is deprived of adequate oxygen supply at the tissue level. Hypoxia may be classified as either generalized, affecting the whole body, or local, affecting a region of the body. Although hypoxia is often a pathological condition, variations in arterial oxygen concentrations can be part of the normal physiology, for example, during strenuous physical exercise.
Dead space is the volume of air that is inhaled that does not take part in the gas exchange, because it either remains in the conducting airways or reaches alveoli that are not perfused or poorly perfused. It means that not all the air in each breath is available for the exchange of oxygen and carbon dioxide. Mammals breathe in and out of their lungs, wasting that part of the inhalation which remains in the conducting airways where no gas exchange can occur.
An arterial blood gas (ABG) test, or arterial blood gas analysis (ABGA) measures the amounts of arterial gases, such as oxygen and carbon dioxide. An ABG test requires that a small volume of blood be drawn from the radial artery with a syringe and a thin needle, but sometimes the femoral artery in the groin or another site is used. The blood can also be drawn from an arterial catheter.

Acetazolamide, sold under the trade name Diamox among others, is a medication used to treat glaucoma, epilepsy, acute mountain sickness, periodic paralysis, idiopathic intracranial hypertension, heart failure and to alkalinize urine. It may be used long term for the treatment of open angle glaucoma and short term for acute angle closure glaucoma until surgery can be carried out. It is taken by mouth or injection into a vein. Acetazolamide is a first generation carbonic anhydrase inhibitor and it decreases the ocular fluid and osmolality in the eye to decrease intraocular pressure.
Acidosis is a biological process producing hydrogen ions and increasing their concentration in blood or body fluids. pH is the negative log of hydrogen ion concentration and so it is decreased by a process of acidosis.
In chemistry and biochemistry, the Henderson–Hasselbalch equation relates the pH of a chemical solution of a weak acid to the numerical value of the acid dissociation constant, Ka, of acid and the ratio of the concentrations, of the acid and its conjugate base in an equilibrium.

Gastric acid or stomach acid is the acidic component – hydrochloric acid of gastric juice, produced by parietal cells in the gastric glands of the stomach lining. With a pH of between one and three, gastric acid plays a key role in the digestion of proteins by activating digestive enzymes, which together break down the long chains of amino acids of proteins. Gastric acid is regulated in feedback systems to increase production when needed, such as after a meal. Other cells in the stomach produce bicarbonate, a base, to buffer the fluid, ensuring a regulated pH. These cells also produce mucus – a viscous barrier to prevent gastric acid from damaging the stomach. The pancreas further produces large amounts of bicarbonate and secretes bicarbonate through the pancreatic duct to the duodenum to neutralize gastric acid passing into the digestive tract.

Respiratory acidosis is a state in which decreased ventilation (hypoventilation) increases the concentration of carbon dioxide in the blood and decreases the blood's pH.
In physiology, base excess and base deficit refer to an excess or deficit, respectively, in the amount of base present in the blood. The value is usually reported as a concentration in units of mEq/L (mmol/L), with positive numbers indicating an excess of base and negative a deficit. A typical reference range for base excess is −2 to +2 mEq/L.
Carbaminohemoglobin (carbaminohaemoglobin BrE) (CO2Hb, also known as carbhemoglobin and carbohemoglobin) is a compound of hemoglobin and carbon dioxide, and is one of the forms in which carbon dioxide exists in the blood. Twenty-three percent of carbon dioxide is carried in blood this way (70% is converted into bicarbonate by carbonic anhydrase and then carried in plasma, 7% carried as free CO2, dissolved in plasma).
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. Vice versa, it is true that a high concentration of CO2 facilitates dissociation of oxyhemoglobin, though this is the result of two distinct processes (Bohr effect and Margaria-Green effect) and should be distinguished from Haldane effect.
Carbamino refers to an adduct generated by the addition of carbon dioxide to the free amino group of an amino acid or a protein, such as hemoglobin forming carbaminohemoglobin.
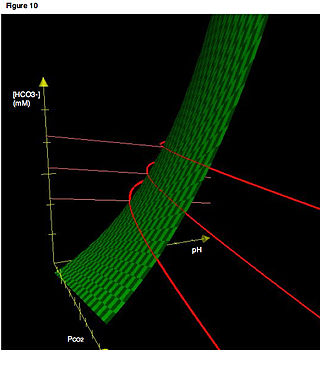
In acid base physiology, the Davenport diagram is a graphical tool, developed by Horace W. Davenport, that allows a clinician or investigator to describe blood bicarbonate concentrations and blood pH following a respiratory and/or metabolic acid-base disturbance. The diagram depicts a three-dimensional surface describing all possible states of chemical equilibria between gaseous carbon dioxide, aqueous bicarbonate and aqueous protons at the physiologically complex interface of the alveoli of the lungs and the alveolar capillaries. Although the surface represented in the diagram is experimentally determined, the Davenport diagram is rarely used in the clinical setting, but allows the investigator to envision the effects of physiological changes on blood acid-base chemistry. For clinical use there are two recent innovations: an Acid-Base Diagram which provides Text Descriptions for the abnormalities and a High Altitude Version that provides text descriptions appropriate for the altitude.

The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid (H2CO3), bicarbonate ion (HCO−
3), and carbon dioxide (CO2) in order to maintain pH in the blood and duodenum, among other tissues, to support proper metabolic function. Catalyzed by carbonic anhydrase, carbon dioxide (CO2) reacts with water (H2O) to form carbonic acid (H2CO3), which in turn rapidly dissociates to form a bicarbonate ion (HCO−
3 ) and a hydrogen ion (H+) as shown in the following reaction:
The Alveolar–arterial gradient, is a measure of the difference between the alveolar concentration (A) of oxygen and the arterial (a) concentration of oxygen. It is a useful parameter for narrowing the differential diagnosis of hypoxemia.
Winters's formula, named after R. W. Winters, is a formula used to evaluate respiratory compensation when analyzing acid-base disorders in the presence of metabolic acidosis. It can be given as:
In some individuals, the effect of oxygen on chronic obstructive pulmonary disease is to cause increased carbon dioxide retention,

The carbonic anhydrases form a family of enzymes that catalyze the interconversion between carbon dioxide and water and the dissociated ions of carbonic acid. The active site of most carbonic anhydrases contains a zinc ion. They are therefore classified as metalloenzymes. The enzyme maintains acid-base balance and helps transport carbon dioxide.

The intestinal mucosal barrier, also referred to as intestinal barrier, refers to the property of the intestinal mucosa that ensures adequate containment of undesirable luminal contents within the intestine while preserving the ability to absorb nutrients. The separation it provides between the body and the gut prevents the uncontrolled translocation of luminal contents into the body proper. Its role in protecting the mucosal tissues and circulatory system from exposure to pro-inflammatory molecules, such as microorganisms, toxins, and antigens is vital for the maintenance of health and well-being. Intestinal mucosal barrier dysfunction has been implicated in numerous health conditions such as: food allergies, microbial infections, irritable bowel syndrome, inflammatory bowel disease, celiac disease, metabolic syndrome, non-alcoholic fatty liver disease, diabetes, and septic shock.








