In chemistry, a hydrogen bond is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative "donor" atom or group (Dn), and another electronegative atom bearing a lone pair of electrons—the hydrogen bond acceptor (Ac). Such an interacting system is generally denoted Dn−H···Ac, where the solid line denotes a polar covalent bond, and the dotted or dashed line indicates the hydrogen bond. The most frequent donor and acceptor atoms are the period 2 elements nitrogen (N), oxygen (O), and fluorine (F).
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects.

In chemistry, a supramolecular assembly is a complex of molecules held together by noncovalent bonds. While a supramolecular assembly can be simply composed of two molecules, or a defined number of stoichiometrically interacting molecules within a quaternary complex, it is more often used to denote larger complexes composed of indefinite numbers of molecules that form sphere-, rod-, or sheet-like species. Colloids, liquid crystals, biomolecular condensates, micelles, liposomes and biological membranes are examples of supramolecular assemblies, and their realm of study is known as supramolecular chemistry. The dimensions of supramolecular assemblies can range from nanometers to micrometers. Thus they allow access to nanoscale objects using a bottom-up approach in far fewer steps than a single molecule of similar dimensions.
In crystallography, polymorphism describes the phenomenon where a compound or element can crystallize into more than one crystal structure. The preceding definition has evolved over many years and is still under discussion today. Discussion of the defining characteristics of polymorphism involves distinguishing among types of transitions and structural changes occurring in polymorphism versus those in other phenomena.
Jack David Dunitz FRS was a British chemist and widely known chemical crystallographer. He was Professor of Chemical Crystallography at the ETH Zurich from 1957 until his official retirement in 1990. He held Visiting Professorships in the United States, Israel, Japan, Canada, Spain and the United Kingdom.
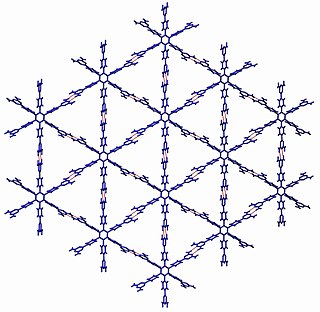
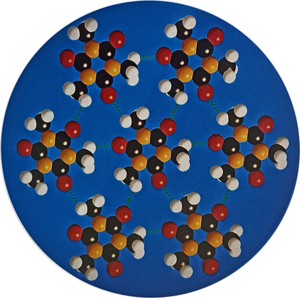
Crystal engineering studies the design and synthesis of solid-state structures with desired properties through deliberate control of intermolecular interactions. It is an interdisciplinary academic field, bridging solid-state and supramolecular chemistry.

CrystEngComm is a peer-reviewed online-only scientific journal publishing original research and review articles on all aspects of crystal engineering including properties, polymorphism, target materials, and crystalline nanomaterials. It is published biweekly by the Royal Society of Chemistry and the editor-in-chief is Pierangelo Metrangolo. According to the Journal Citation Reports, the journal has a 2021 impact factor of 3.756. CrystEngComm has a close association with the virtual web community, CrystEngCommunity.

A molecular solid is a solid consisting of discrete molecules. The cohesive forces that bind the molecules together are van der Waals forces, dipole–dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, halogen bonding, London dispersion forces, and in some molecular solids, coulombic interactions. Van der Waals, dipole interactions, quadrupole interactions, π–π interactions, hydrogen bonding, and halogen bonding are typically much weaker than the forces holding together other solids: metallic, ionic, and network solids. Intermolecular interactions typically do not involve delocalized electrons, unlike metallic and certain covalent bonds. Exceptions are charge-transfer complexes such as the tetrathiafulvane-tetracyanoquinodimethane (TTF-TCNQ), a radical ion salt. These differences in the strength of force and electronic characteristics from other types of solids give rise to the unique mechanical, electronic, and thermal properties of molecular solids.
In chemistry, a halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. Like a hydrogen bond, the result is not a formal chemical bond, but rather a strong electrostatic attraction. Mathematically, the interaction can be decomposed in two terms: one describing an electrostatic, orbital-mixing charge-transfer and another describing electron-cloud dispersion. Halogen bonds find application in supramolecular chemistry; drug design and biochemistry; crystal engineering and liquid crystals; and organic catalysis.
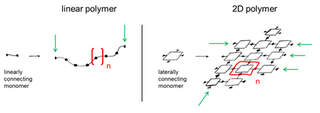
A two-dimensional polymer (2DP) is a sheet-like monomolecular macromolecule consisting of laterally connected repeat units with end groups along all edges. This recent definition of 2DP is based on Hermann Staudinger's polymer concept from the 1920s. According to this, covalent long chain molecules ("Makromoleküle") do exist and are composed of a sequence of linearly connected repeat units and end groups at both termini.

Ayyappanpillai Ajayaghosh is a research scientist/academician in the domain of interdisciplinary chemistry, and the former Director of the National Institute for Interdisciplinary Science and Technology. He is known for his studies on supramolecular assemblies, organogels, photoresponsive materials, chemosensory and security materials systems and is an elected fellow of all the three major Indian science academies viz. the National Academy of Sciences, India, Indian National Science Academy and the Indian Academy of Sciences as well as The World Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards for his contributions to Chemical Sciences in 2007. He is the first chemist to receive the Infosys Science Prize for physical sciences, awarded by the Infosys Science Foundation. He received the TWAS Prize of The World Academy of Sciences in 2013 and the Goyal prize in 2019.

Harry Laurence Anderson is a British chemist in the Department of Chemistry, University of Oxford. He is well known for his contributions in the syntheses of supramolecular systems, exploration of the extraordinary physical properties of large pi-conjugated systems, and synthesis of cyclo[18]carbon. He is a Professor of Chemistry at Keble College, Oxford.
Pierangelo Metrangolo is an Italian chemist with interests in supramolecular chemistry and functional materials. He also has an interest in crystal engineering, in particular by using the halogen bond. He is Vice-President and President-Elect of the Physical and Biophysical Chemistry Division of IUPAC.
Partha Sarathi Mukherjee is an Indian inorganic chemist and a professor at the Inorganic and Physical Chemistry department of the Indian Institute of Science. He is known for his studies on organic nano structures, molecular sensors and catalysis in nanocages. He is a recipient of the Swarnajayanthi Fellowship of the Department of Science and Technology and the Bronze Medal of the Chemical Research Society of India. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 2016, for his contributions to chemical sciences.

Subi Jacob George is an Indian organic chemist, known for his work in the fields of supramolecular chemistry, materials chemistry, and polymer chemistry. His research interests includes organic and supramolecular synthesis, functional organic materials, supramolecular polymers, chiral amplification, hybrid materials, and optoelectronic materials.

Margaret Cairns Etter, known informally as Peggy Etter, was an American chemist who contributed to the development of solid state chemistry for crystalline organic compounds. She is known for her work characterizing and classifying contacts by hydrogen bonds in organic compounds. Her "enlightened imagination, innovative creativity, and unfailing enthusiasm" is recognised as having a "transformative effect" in many areas of organic chemistry.
Rahul Banerjee is a Bengali Indian organic chemist and a professor at the department of chemical sciences of the Indian Institute of Science Education and Research, Kolkata. Banerjee, a fellow of the Royal Society of Chemistry, is known for his studies in the field of Metal–organic framework designing. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, for his contributions to chemical sciences in 2018. Currently he is one of the associate editor of international peer-review journal Journal of the American Chemical Society.

Dorothy June Sutor was a New Zealand-born crystallographer who spent most of her research career in England. She was one of the first scientists to establish that hydrogen bonds could form to hydrogen atoms bonded to carbon atoms. She later worked in the laboratory of Kathleen Lonsdale on the characterisation and prevention of urinary calculi.
Nathan C. Gianneschi is the Jacob & Rosaline Cohn Professor of Chemistry, Materials Science & Engineering, and Biomedical Engineering at Northwestern University and the Associate Director for the International Institute for Nanotechnology. Gianneschi's lab takes an interdisciplinary approach to nanomaterials research, with a focus on multifunctional materials for biomedical applications, programmed interactions with biomolecules and cells, and basic research into nanoscale materials design, synthesis and characterization.

Kumar Biradha is a researcher in the field of crystal engineering. He was born on 15 June 1968 in Relangi, Andhra Pradesh. He is a professor at the Department of Chemistry, Indian Institute of Technology, Kharagpur, and a member of Editorial Advisory board of Crystal Growth & Design, an American Chemical Society Journal.