
A pilus is a hair-like appendage found on the surface of many bacteria and archaea. The terms pilus and fimbria can be used interchangeably, although some researchers reserve the term pilus for the appendage required for bacterial conjugation. All conjugative pili are primarily composed of pilin – fibrous proteins, which are oligomeric.
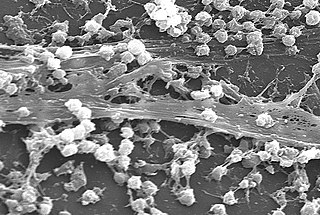
A biofilm comprises any syntrophic consortium of microorganisms in which cells stick to each other and often also to a surface. These adherent cells become embedded within a slimy extracellular matrix that is composed of extracellular polymeric substances (EPSs). The cells within the biofilm produce the EPS components, which are typically a polymeric conglomeration of extracellular polysaccharides, proteins, lipids and DNA. Because they have three-dimensional structure and represent a community lifestyle for microorganisms, they have been metaphorically described as "cities for microbes".

Vibrio cholerae is a species of Gram-negative, facultative anaerobe and comma-shaped bacteria. The bacteria naturally live in brackish or saltwater where they attach themselves easily to the chitin-containing shells of crabs, shrimps, and other shellfish. Some strains of V. cholerae are pathogenic to humans and cause a deadly disease cholera, which can be derived from the consumption of undercooked or raw marine life species.

Geobacter is a genus of bacteria. Geobacter species are anaerobic respiration bacterial species which have capabilities that make them useful in bioremediation. Geobacter was found to be the first organism with the ability to oxidize organic compounds and metals, including iron, radioactive metals, and petroleum compounds into environmentally benign carbon dioxide while using iron oxide or other available metals as electron acceptors. Geobacter species are also found to be able to respire upon a graphite electrode. They have been found in anaerobic conditions in soils and aquatic sediment.
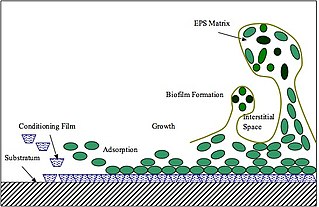
Extracellular polymeric substances (EPSs) are natural polymers of high molecular weight secreted by microorganisms into their environment. EPSs establish the functional and structural integrity of biofilms, and are considered the fundamental component that determines the physicochemical properties of a biofilm. EPS in the matrix of biofilms provides compositional support and protection of microbial communities from the harsh environments. Components of EPS can be of different classes of polysaccharides, lipids, nucleic acids, proteins, Lipopolysaccharides, and minerals.

Shewanella oneidensis is a bacterium notable for its ability to reduce metal ions and live in environments with or without oxygen. This proteobacterium was first isolated from Lake Oneida, NY in 1988, hence its name.

Bacterial nanowires are electrically conductive appendages produced by a number of bacteria most notably from the Geobacter and Shewanella genera. Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus. From physiological and functional perspectives, bacterial nanowires are diverse. The precise role microbial nanowires play in their biological systems has not been fully realized, but several proposed functions exist. Outside of a naturally occurring environment, bacterial nanowires have shown potential to be useful in several fields, notably the bioenergy and bioremediation industries.
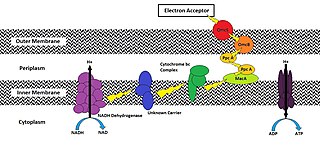
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.
Roberto Kolter is Professor of Microbiology, Emeritus at Harvard Medical School, an author, and past president of the American Society for Microbiology. Kolter has been a professor at Harvard Medical School since 1983 and was Co-director of Harvard's Microbial Sciences Initiative from 2003-2018. During the 35-year term of the Kolter laboratory from 1983 to 2018, more than 130 graduate student and postdoctoral trainees explored an eclectic mix of topics gravitating around the study of microbes. Kolter is a fellow of the American Association for the Advancement of Science and of the American Academy of Microbiology.
Microbial electrosynthesis (MES) is a form of microbial electrocatalysis in which electrons are supplied to living microorganisms via a cathode in an electrochemical cell by applying an electric current. The electrons are then used by the microorganisms to reduce carbon dioxide to yield industrially relevant products. The electric current would ideally be produced by a renewable source of power. This process is the opposite to that employed in a microbial fuel cell, in which microorganisms transfer electrons from the oxidation of compounds to an anode to generate an electric current.
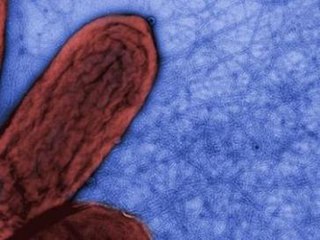
Geobacter sulfurreducens is a gram-negative metal and sulphur-reducing proteobacterium. It is rod-shaped, obligately anaerobic, non-fermentative, has flagellum and type four pili, and is closely related to Geobacter metallireducens. Geobacter sulfurreducens is an anaerobic species of bacteria that comes from the family of bacteria called Geobacteraceae. Under the genus of Geobacter, G. sulfurreducens is one out of twenty different species. The Geobacter genus was discovered by Derek R. Lovley in 1987. G. sulfurreducens was first isolated in Norman, Oklahoma, USA from materials found around the surface of a contaminated ditch.
Geopsychrobacter electrodiphilus is a species of bacteria, the type species of its genus. It is a psychrotolerant member of its family, capable of attaching to the anodes of sediment fuel cells and harvesting electricity by oxidation of organic compounds to carbon dioxide and transferring the electrons to the anode.
Geobacter psychrophilus is a Fe(III)-reducing bacterium. It is Gram-negative, slightly curved, rod-shaped and motile via means of monotrichous flagella. Its type strain is P35T.
OmcS nanowires are conductive filaments found in some species of bacteria, including Geobacter sulfurreducens, where they catalyze the transfer of electrons. They are multiheme c-Type cytochromes localized outside of the cell of some exoelectrogenic bacterial species, serving as mediator of extracellular electron transfer from cells to Fe(III) oxides and other extracellular electron acceptors.
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].

Forest rings are large, circular patterns of low tree density in the boreal forests of northern Canada. These rings can range from 50 metres (160 ft) to nearly 2 kilometres (1.2 mi) in diameter, with rims about 20 metres (66 ft) in thickness. The origin of forest rings is not known, despite several mechanisms for their creation having been proposed. Such hypotheses include radially growing fungus, buried kimberlite pipes, trapped gas pockets, and meteorite impact craters.
Geobacter daltonii is a Gram-negative, Fe(III)- and Uranium(IV)-reducing and non-spore-forming bacterium from the genus of Geobacter. It was isolated from sediments from the Oak Ridge Field Research Center in Oak Ridge, Tennessee in the United States. The specific epithet "daltonii" was refers to Dava Dalton, who performed the initial isolation of the strain, but passed away shortly thereafter.
Geobacter uraniireducens is a gram-negative, rod-shaped, anaerobic, chemolithotrophic, mesophilic, and motile bacterium from the genus of Geobacter. G. uraniireducens has been found to reduce iron and uranium in sediment and soil. It is being studied for use in bioremediation projects due to its ability to reduce uranium and arsenic.

Melanie Blokesch is a German microbiologist. Her research focuses on Vibrio cholerae, the bacterium causing cholera. She is a professor of life sciences at École Polytechnique Fédérale de Lausanne (EPFL), where she heads the Laboratory of Molecular Microbiology.
Microbial electrochemical technologies (METs) use microorganisms as electrochemical catalyst, merging the microbial metabolism with electrochemical processes for the production of bioelectricity, biofuels, H2 and other valuable chemicals. Microbial fuel cells (MFC) and microbial electrolysis cells (MEC) are prominent examples of METs. While MFC is used to generate electricity from organic matter typically associated with wastewater treatment, MEC use electricity to drive chemical reactions such as the production of H2 or methane. Recently, microbial electrosynthesis cells (MES) have also emerged as a promising MET, where valuable chemicals can be produced in the cathode compartment. Other MET applications include microbial remediation cell, microbial desalination cell, microbial solar cell, microbial chemical cell, etc.,.











