
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting bacterial infections, and antibiotic medications are widely used in the treatment and prevention of such infections. They may either kill or inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza; drugs which inhibit growth of viruses are termed antiviral drugs or antivirals rather than antibiotics. They are also not effective against fungi; drugs which inhibit growth of fungi are called antifungal drugs.

Antimicrobial resistance (AMR) occurs when microbes evolve mechanisms that protect them from the effects of antimicrobials. All classes of microbes can evolve resistance where the drugs are no longer effective. Fungi evolve antifungal resistance, viruses evolve antiviral resistance, protozoa evolve antiprotozoal resistance, and bacteria evolve antibiotic resistance. Together all of these come under the umbrella of antimicrobial resistance. Microbes resistant to multiple antimicrobials are called multidrug resistant (MDR) and are sometimes referred to as superbugs. Although antimicrobial resistance is a naturally occurring process, it is often the result of improper usage of the drugs and management of the infections.

Klebsiella pneumoniae is a Gram-negative, non-motile, encapsulated, lactose-fermenting, facultative anaerobic, rod-shaped bacterium. It appears as a mucoid lactose fermenter on MacConkey agar.

Phage therapy, viral phage therapy, or phagotherapy is the therapeutic use of bacteriophages for the treatment of pathogenic bacterial infections. This therapeutic approach emerged at the beginning of the 20th century but was progressively replaced by the use of antibiotics in most parts of the world after the Second World War. Bacteriophages, known as phages, are a form of virus that attach to bacterial cells and inject their genome into the cell. The bacteria's production of the viral genome interferes with its ability to function, halting the bacterial infection. The bacterial cell causing the infection is unable to reproduce and instead produces additional phages. Phages are very selective in the strains of bacteria they are effective against.
Multiple drug resistance (MDR), multidrug resistance or multiresistance is antimicrobial resistance shown by a species of microorganism to at least one antimicrobial drug in three or more antimicrobial categories. Antimicrobial categories are classifications of antimicrobial agents based on their mode of action and specific to target organisms. The MDR types most threatening to public health are MDR bacteria that resist multiple antibiotics; other types include MDR viruses, parasites.

Medical microbiology, the large subset of microbiology that is applied to medicine, is a branch of medical science concerned with the prevention, diagnosis and treatment of infectious diseases. In addition, this field of science studies various clinical applications of microbes for the improvement of health. There are four kinds of microorganisms that cause infectious disease: bacteria, fungi, parasites and viruses, and one type of infectious protein called prion.
The resistome has been used to describe to two similar yet separate concepts:

NDM-1 is an enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotics. These include the antibiotics of the carbapenem family, which are a mainstay for the treatment of antibiotic-resistant bacterial infections. The gene for NDM-1 is one member of a large gene family that encodes beta-lactamase enzymes called carbapenemases. Bacteria that produce carbapenemases are often referred to in the news media as "superbugs" because infections caused by them are difficult to treat. Such bacteria are usually sensitive only to polymyxins and tigecycline.
Plasmid-mediated resistance is the transfer of antibiotic resistance genes which are carried on plasmids. Plasmids possess mechanisms that ensure their independent replication as well as those that regulate their replication number and guarantee stable inheritance during cell division. By the conjugation process, they can stimulate lateral transfer between bacteria from various genera and kingdoms. Numerous plasmids contain addiction-inducing systems that are typically based on toxin-antitoxin factors and capable of killing daughter cells that don't inherit the plasmid during cell division. Plasmids often carry multiple antibiotic resistance genes, contributing to the spread of multidrug-resistance (MDR). Antibiotic resistance mediated by MDR plasmids severely limits the treatment options for the infections caused by Gram-negative bacteria, especially family Enterobacteriaceae. The global spread of MDR plasmids has been enhanced by selective pressure from antimicrobial medications used in medical facilities and when raising animals for food.
Julian Edmund Davies is a British-born microbiologist and Professor Emeritus in the Department of Microbiology and Immunology at the University of British Columbia.

Aspergillomarasmine A is an polyamino acid naturally produced by the mold Aspergillus versicolor. The substance has been reported to inhibit two antibiotic resistance carbapenemase proteins in bacteria, New Delhi metallo-beta-lactamase 1 (NDM-1) and Verona integron-encoded metallo-beta-lactamase (VIM-2), and make those antibiotic-resistant bacteria susceptible to antibiotics. Aspergillomarasmine A is toxic to leaves of barley and other plants, being termed as "Toxin C" when produced by Pyrenophora teres.
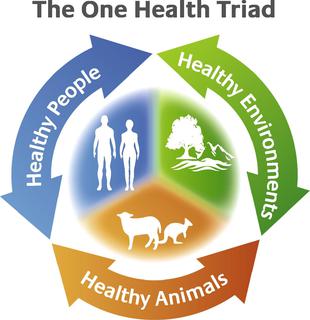
The concept of One Health is the unity of multiple practices that work together locally, nationally, and globally to help achieve optimal health for people, animals, and the environment. When the people, animals, and environment are put together they make up the One Health Triad .The One Health Triad shows how the health of people, animals, and the environment is linked to one another. With One Health being a worldwide concept, it makes it easier to advance health care in the 21st century. When this concept is used, and applied properly, it can help protect people, animals, and the environment in the present and future generations.

Squire Booker is an American biochemist at Penn State University. Booker directs an interdisciplinary chemistry research program related to fields of biochemistry, enzymology, protein chemistry, natural product biosynthesis, and mechanisms of radical dependent enzymes. He is an associate editor for the American Chemical Society Biochemistry Journal, is a Hughes Medical Institute Investigator, and an Eberly Distinguished Chair in Science at Penn State University.
Asad Ullah Khan is an Indian microbiologist, biochemist and a professor at the Interdisciplinary Biotechnology Unit of the Aligarh Muslim University. He is known for his studies on multidrug resistant clinical strains as well as for the first sighting in India of Aligarh super bug (NDM-4), a variant of New Delhi metallo-beta-lactamase 1 (NDM-1). He is an elected fellow of the Royal Society of Chemistry, the Biotech Research Society, India and the Indian Academy of Microbiological Sciences. The Department of Biotechnology of the Government of India awarded him the National Bioscience Award for Career Development, one of the highest Indian science awards, for his contributions to biosciences, in 2012.
Houra Merrikh is an Iranian-American microbiologist. She is a full professor at Vanderbilt University in the Department of Biochemistry. Her field of work is antibiotic resistance and bacterial evolvability.
Helen Boucher is Dean of Tufts University School of Medicine and Chief Academic Officer of Tufts Medicine, the parent health system for Tufts Medical Center in Boston. Prior to this, she served as Chief of the Division of Geographic Medicine and Infectious Diseases at Tufts Medical Center, a Professor of Medicine at Tufts University School of Medicine, and Director of the Stuart B. Levy Center for Integrated Management of Antimicrobial Resistance at Tufts.

Georg Peters was a German physician, microbiologist and university professor. From 1992 until his fatal mountain accident he headed the Institute of Medical Microbiology at the University of Münster. He was an internationally recognised expert in the field of staphylococci and the infectious diseases caused by them, to which he had devoted himself since the beginning of his scientific career.
Laura Piddock is a microbiologist, specialising in antibiotics and antibiotic resistance in bacteria. She is Professor Emerita at the University of Birmingham, UK and also Scientific Director within the Global Antibiotic Research and Development Partnership.
Lori Lee Burrows is a Canadian microbiologist. She is a Tier 1 Canada Research Chair in Microbe-Surface Interactions at McMaster University.









