 | |
| Names | |
|---|---|
| IUPAC name (2S)-2-[[(2R)-2-[[(2R)-2-amino-2-carboxyethyl]amino]-2-carboxyethyl]amino]butanedioic acid | |
| Other names Toxin C | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
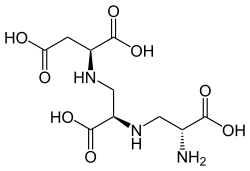
| C10H17N3O8 | |
| Molar mass | 307.259 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Aspergillomarasmine A is an polyamino acid naturally produced by the mold Aspergillus versicolor . The substance has been reported to inhibit two antibiotic resistance carbapenemase proteins in bacteria, New Delhi metallo-beta-lactamase 1 (NDM-1) and Verona integron-encoded metallo-beta-lactamase (VIM-2), and make those antibiotic-resistant bacteria susceptible to antibiotics. [1] Aspergillomarasmine A is toxic to leaves of barley and other plants, being termed as "Toxin C" when produced by Pyrenophora teres . [2]
The molecule is a tetracarboxylic acid with four -COOH groups. One section of the molecule is the amino acid aspartic acid. This has two alanine [ contradictory ] molecules attached by substituting a hydrogen on the methyl group with a link to the amine group. Aspergillomarasmine B differs in that the last alanine is replaced by glycine.
The crystalline substance was first isolated in 1956, but its name was given until 1965. [3]
In addition to Aspergillus versicolor, aspergillomarasmine A is also produced by the ascomycete Pyrenophora teres where it acts as a toxin in the barley net-spot blotch disease. In P. teres, a biosynthetic precursor of aspergillomarasmine A, L,L-N-(2-amino-2-carboxyethyl)-aspartic acid has also been isolated and found to contribute to the phytotoxic properties of this microbe. [4] This precursor, aspergillomarasmine A itself, and a lactam form (anhydroaspergillomarasmine A) are together termed the marasmines. [2]
Other producers of aspergillomarasmine A include Aspergillus flavus , [3] Aspergillus oryzae , [5] Colletotrichum gloeosporioides , and Fusarium oxysporum . [2]
In mice, the LD50 toxic dose of aspergillomarasmine A is 159.8 mg/kg. [6]