
A mold or mould is one of the structures that certain fungi can form. The dust-like, colored appearance of molds is due to the formation of spores containing fungal secondary metabolites. The spores are the dispersal units of the fungi. Not all fungi form molds. Some fungi form mushrooms; others grow as single cells and are called microfungi.
A mycotoxin is a toxic secondary metabolite produced by fungi and is capable of causing disease and death in both humans and other animals. The term 'mycotoxin' is usually reserved for the toxic chemical products produced by fungi that readily colonize crops.
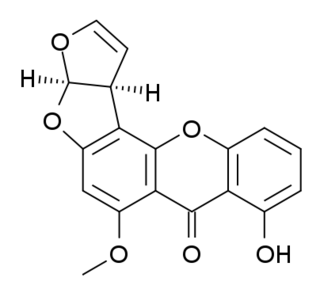
Sterigmatocystin is a polyketide mycotoxin produced by certain species of Aspergillus. The toxin is naturally found in some cheeses.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Mucor racemosus is a rapidly growing, weedy mould belonging to the division Mucoromycota. It is one of the earliest fungi to be grown in pure culture and was first isolated in 1886. It has a worldwide distribution and colonizes many habitats such as vegetational products, soil and houses. The fungus is mostly known for its ability to exhibit both filamentous and yeast-like morphologies, often referred to as dimorphism. Stark differences are seen in both forms and conditions of the environment heavily affect the phases of the M. racemosus. Like many fungi, it also reproduces both sexually and asexually. The dimorphic capacity of this species has been proposed as an important factor in its pathogenicity and has enhanced the industrial importance. This species is considered an opportunistic pathogen, generally limited to immunocompromised individuals. It also been associated with allergy and inflammations of facial sinuses. Its association with allergy has made it a common fungus used in allergen medical testing. Industrial use of the fungus is in the production of enzymes and the manufacture of certain dairy foods.
Aspergillus ochraceus is a mold species in the genus Aspergillus known to produce the toxin ochratoxin A, one of the most abundant food-contaminating mycotoxins, and citrinin. It also produces the dihydroisocoumarin mellein. It is a filamentous fungus in nature and has characteristic biseriate conidiophores. Traditionally a soil fungus, has now began to adapt to varied ecological niches, like agricultural commodities, farmed animal and marine species. In humans and animals the consumption of this fungus produces chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects. Its airborne spores are one of the potential causes of asthma in children and lung diseases in humans. The pig and chicken populations in the farms are the most affected by this fungus and its mycotoxins. Certain fungicides like mancozeb, copper oxychloride, and sulfur have inhibitory effects on the growth of this fungus and its mycotoxin producing capacities.
Aspergillus sydowii is a pathogenic fungus that causes several diseases in humans. It has been implicated in the death of sea fan corals in the Caribbean Sea.
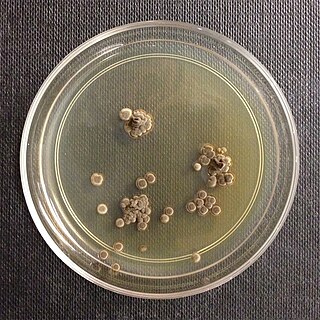
Wallemia sebi is a xerophilic fungus of the phylum Basidiomycota.
Aspergillus penicillioides is a species of fungus in the genus Aspergillus, and is among the most xerophilic fungi.
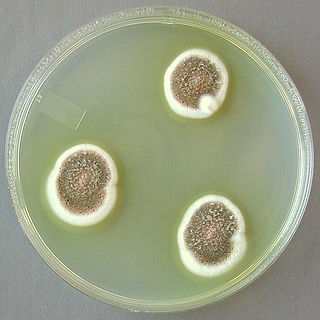
Aspergillus ustus is a microfungus and member of the division Ascomycota. It is commonly found in indoor environments and soil. Isolated cases of human infection resulting from A. ustus have been described; however the majority of these are nail infections.

Aspergillus glaucus is a filamentous fungus which is known to have a wide environmental distribution due to its physiological hardiness under extreme conditions. Like many other fungi belonging to the genus Aspergillus, it can be mildly pathogenic but has a number of useful potential applications in medicine and the production of foodstuffs.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.

Aspergillus clavatus is a species of fungus in the genus Aspergillus with conidia dimensions 3–4.5 x 2.5–4.5 μm. It is found in soil and animal manure. The fungus was first described scientifically in 1834 by the French mycologist John Baptiste Henri Joseph Desmazières.
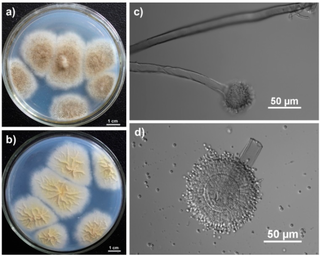
Aspergillus tubingensis is a darkly pigmented species of fungus in the genus Aspergillus section Nigri. It is often confused with Aspergillus niger due to their similar morphology and habitat. A. tubingensis is often involved in food spoilage of fruits and wheat, and industrial fermentation. This species is a rare agent of opportunistic infection.

Aspergillus parasiticus is a fungus belonging to the genus Aspergillus. This species is an unspecialized saprophytic mold, mostly found outdoors in areas of rich soil with decaying plant material as well as in dry grain storage facilities. Often confused with the closely related species, A. flavus, A. parasiticus has defined morphological and molecular differences. Aspergillus parasiticus is one of three fungi able to produce the mycotoxin, aflatoxin, one of the most carcinogenic naturally occurring substances. Environmental stress can upregulate aflatoxin production by the fungus, which can occur when the fungus is growing on plants that become damaged due to exposure to poor weather conditions, during drought, by insects, or by birds. In humans, exposure to A. parasiticus toxins can cause delayed development in children and produce serious liver diseases and/or hepatic carcinoma in adults. The fungus can also cause the infection known as aspergillosis in humans and other animals. A. parasiticus is of agricultural importance due to its ability to cause disease in corn, peanut, and cottonseed.
Ulocladium botrytis is an anamorphic filamentous fungus belonging to the phylum Ascomycota. Commonly found in soil and damp indoor environments, U.botrytis is a hyphomycetous mould found in many regions of the world. It is also occasionally misidentified as a species of the genera Alternaria or Pithomyces due to morphological similarities. Ulocladium botrytis is rarely pathogenic to humans but is associated with human allergic responses and is used in allergy tests. Ulocladium botrytis has been implicated in some cases of human fungal nail infection. The fungus was first discovered in 1851 by German mycologist Carl Gottlieb Traugott Preuss.
Aspergillus wentii is an asexual, filamentous, endosymbiotic fungus belonging to the mold genus, Aspergillus. It is a common soil fungus with a cosmopolitan distribution, although it is primarily found in subtropical regions. Found on a variety of organic materials, A. wentii is known to colonize corn, cereals, moist grains, peanuts and other ground nut crops. It is also used in the manufacture of biodiesel from lipids and is known for its ability to produce enzymes used in the food industry.
Botryotrichum piluliferum is a fungal species first identified in 1885 by Saccardo and Marchal. It was discovered to be the asexual state of a member of the ascomycete genus, Chaetomium. The name B. piluliferum now applies to the fungus in all its states. B. piluliferum has been found worldwide in a wide range of habitats such as animal dung and vegetation. The colonies of this fungus start off white and grow rapidly to a brown colour. The conidia are smooth and white. B. piluliferum grows optimally at a temperature of 25-30 °C and a pH of 5.5.
Aspergillus giganteus is a species of fungus in the genus Aspergillus that grows as a mold. It was first described in 1901 by Wehmer, and is one of six Aspergillus species from the Clavati section of the subgenus Fumigati. Its closest taxonomic relatives are Aspergillus rhizopodus and Aspergillus longivescia.
Aspergillus carneus is a fast-growing, filamentous fungus found on detritus and in fertile soil worldwide. It is characterized by its yellow, thick-walled hyphae and biseriate sterigmata. The fungus produces citrinin and 5 unique depsipeptides, Aspergillicins A-E.










