
Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defining feature of this fungal group is the "ascus", a microscopic sexual structure in which nonmotile spores, called ascospores, are formed. However, some species of Ascomycota are asexual and thus do not form asci or ascospores. Familiar examples of sac fungi include morels, truffles, brewers' and bakers' yeast, dead man's fingers, and cup fungi. The fungal symbionts in the majority of lichens such as Cladonia belong to the Ascomycota.

Germination is the process by which an organism grows from a seed or spore. The term is applied to the sprouting of a seedling from a seed of an angiosperm or gymnosperm, the growth of a sporeling from a spore, such as the spores of fungi, ferns, bacteria, and the growth of the pollen tube from the pollen grain of a seed plant.

Aspergillus flavus is a saprotrophic and pathogenic fungus with a cosmopolitan distribution. It is best known for its colonization of cereal grains, legumes, and tree nuts. Postharvest rot typically develops during harvest, storage, and/or transit. Its specific name flavus derives from the Latin meaning yellow, a reference to the frequently observed colour of the spores. A. flavus infections can occur while hosts are still in the field (preharvest), but often show no symptoms (dormancy) until postharvest storage or transport. In addition to causing preharvest and postharvest infections, many strains produce significant quantities of toxic compounds known as mycotoxins, which, when consumed, are toxic to mammals. A. flavus is also an opportunistic human and animal pathogen, causing aspergillosis in immunocompromised individuals.

Aspergillus fumigatus is a species of fungus in the genus Aspergillus, and is one of the most common Aspergillus species to cause disease in individuals with an immunodeficiency.

Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.

Colletotrichum lindemuthianum is a fungus which causes anthracnose, or black spot disease, of the common bean plant. It is considered a hemibiotrophic pathogen because it spends part of its infection cycle as a biotroph, living off of the host but not harming it, and the other part as a necrotroph, killing and obtaining nutrients from the host tissues.
Nigrospora sphaerica is an airborne filamentous fungus in the phylum Ascomycota. It is found in soil, air, and plants as a leaf pathogen. It can occur as an endophyte where it produces antiviral and antifungal secondary metabolites. Sporulation of N. sphaerica causes its initial white coloured colonies to rapidly turn black. N. sphaerica is often confused with the closely related species N. oryzae due to their morphological similarities.
Conidial anastomosis tubes (CATs) are cells formed from the conidia of many filamentous fungi. These cells have a tubular shape and form an anastomosis (bridge) that allows fusion between conidia.
Aspergillus ochraceus is a mold species in the genus Aspergillus known to produce the toxin ochratoxin A, one of the most abundant food-contaminating mycotoxins, and citrinin. It also produces the dihydroisocoumarin mellein. It is a filamentous fungus in nature and has characteristic biseriate conidiophores. Traditionally a soil fungus, has now began to adapt to varied ecological niches, like agricultural commodities, farmed animal and marine species. In humans and animals the consumption of this fungus produces chronic neurotoxic, immunosuppressive, genotoxic, carcinogenic and teratogenic effects. Its airborne spores are one of the potential causes of asthma in children and lung diseases in humans. The pig and chicken populations in the farms are the most affected by this fungus and its mycotoxins. Certain fungicides like mancozeb, copper oxychloride, and sulfur have inhibitory effects on the growth of this fungus and its mycotoxin producing capacities.
Aspergillus penicillioides is a species of fungus in the genus Aspergillus, and is among the most xerophilic fungi.
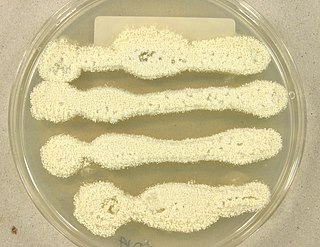
Aspergillus candidus is a white-spored species of fungus in the genus Aspergillus. Despite its lack of pigmentation, it is closely related to the most darkly-pigmented aspergilli in the Aspergillus niger group. It is a common soil fungus worldwide and is known as a contaminant of a wide array of materials from the indoor environment to foods and products. It is an uncommon agent of onychomycosis and aspergillosis. The species epithet candidus (L.) refers to the white pigmentation of colonies of this fungus. It is from the Candidi section. The fungi in the Candidi section are known for their white spores. It has been isolated from wheat flour, djambee, and wheat grain.

Penicillium digitatum is a mesophilic fungus found in the soil of citrus-producing areas. It is a major source of post-harvest decay in fruits and is responsible for the widespread post-harvest disease in Citrus fruit known as green rot or green mould. In nature, this necrotrophic wound pathogen grows in filaments and reproduces asexually through the production of conidiophores and conidia. However, P. digitatum can also be cultivated in the laboratory setting. Alongside its pathogenic life cycle, P. digitatum is also involved in other human, animal and plant interactions and is currently being used in the production of immunologically based mycological detection assays for the food industry.

Aspergillus clavatus is a species of fungus in the genus Aspergillus with conidia dimensions 3–4.5 x 2.5–4.5 μm. It is found in soil and animal manure. The fungus was first described scientifically in 1834 by the French mycologist John Baptiste Henri Joseph Desmazières.

Penicillium spinulosum is a non-branched, fast-growing fungus with a swelling at the terminal of the stipe (vesiculate) in the genus Penicillium. P. spinulosum is able to grow and reproduce in environment with low temperature and low water availability, and is known to be acidotolerant. P. spinulosum is ubiquitously distributed, and can often be isolated from soil. Each individual strain of P. spinulosum differs from others in their colony morphology, including colony texture, amount of sporulation and roughness of conidia and conidiophores.
Aspergillus wentii is an asexual, filamentous, endosymbiotic fungus belonging to the mold genus, Aspergillus. It is a common soil fungus with a cosmopolitan distribution, although it is primarily found in subtropical regions. Found on a variety of organic materials, A. wentii is known to colonize corn, cereals, moist grains, peanuts and other ground nut crops. It is also used in the manufacture of biodiesel from lipids and is known for its ability to produce enzymes used in the food industry.

Alternaria brassicicola is a fungal necrotrophic plant pathogen that causes black spot disease on a wide range of hosts, particularly in the genus of Brassica, including a number of economically important crops such as cabbage, Chinese cabbage, cauliflower, oilseeds, broccoli and canola. Although mainly known as a significant plant pathogen, it also contributes to various respiratory allergic conditions such as asthma and rhinoconjunctivitis. Despite the presence of mating genes, no sexual reproductive stage has been reported for this fungus. In terms of geography, it is most likely to be found in tropical and sub-tropical regions, but also in places with high rain and humidity such as Poland. It has also been found in Taiwan and Israel. Its main mode of propagation is vegetative. The resulting conidia reside in the soil, air and water. These spores are extremely resilient and can overwinter on crop debris and overwintering herbaceous plants.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.
Aspergillus giganteus is a species of fungus in the genus Aspergillus that grows as a mold. It was first described in 1901 by Wehmer, and is one of six Aspergillus species from the Clavati section of the subgenus Fumigati. Its closest taxonomic relatives are Aspergillus rhizopodus and Aspergillus longivescia.

This glossary of mycology is a list of definitions of terms and concepts relevant to mycology, the study of fungi. Terms in common with other fields, if repeated here, generally focus on their mycology-specific meaning. Related terms can be found in glossary of biology and glossary of botany, among others. List of Latin and Greek words commonly used in systematic names and Botanical Latin may also be relevant, although some prefixes and suffixes very common in mycology are repeated here for clarity.
Meristacrum is a fungal genus in the monotypic family Meristacraceae, of the order Entomophthorales. They are parasites of soil invertebrates, they typically infect nematodes, and tardigrades.














