
Giuseppe Di Giovanni, Cavaliere (born May 24, 1968; Palermo, Italy) is a Professor of Human Physiology and Neuroscience at the University of Malta.

Giuseppe Di Giovanni, Cavaliere (born May 24, 1968; Palermo, Italy) is a Professor of Human Physiology and Neuroscience at the University of Malta.
Di Giovanni Giuseppe received his PhD in Neuroscience from the University of Chieti, Italy and was a postdoctoral fellow at Yale University, CT, USA.
He served as a Senior Lecturer of Human Physiology at the Faculty of Medicine and Surgery, University of Palermo. Later he became Professor of Human Physiology and Neuroscience at the University of Malta, as well as an Honorary Professor at the Neuroscience Division of the School Biosciences at Cardiff University, UK. [1] His main research interests are in experimental neurology and biological psychiatry. Specifically, he is interested in the pathophysiological role of cannabinoids and serotonin, and especially of the 5-HT2C receptors, in brain disorders, such as epilepsy, depression, drugs of abuse and Parkinson's disease. He has published more than 150 articles in top biomedical journals including Nature Medicine and Nature Neuroscience, 15 books and several journal special issues. [2] [3] [4] [5] [6] [7] [8] [9] He is the President of the Mediterranean Neuroscience Society (MNS), the President of the Malta Physiological Society and the Treasurer of the Malta Neuroscience Network. He is the Editor-in-Chief of the prestigious Journal of Neuroscience Methods by Elsevier, Amsterdam, Netherlands and the Editor of the book series "The Receptors" by Springer, USA and serves as associate editor for the CNS Neuroscience & Therapeutics by Wiley. On 28 May 2020 he was awarded the honour of Cavaliere (Knight) of the Order of the Star of Italy by the President of the Italian Republic Sergio Mattarella. He was listed among the World’s Top 2% Scientists ranking for his work in 2019 [10] and 2020 [11] in the field of Neuroscience. In 2022, he was elected a member of Academia Europaea, due to his achievements in Physiology and Neuroscience research and Secretary-General of IBRO, the International Brain Research Organization, for the term January 2023 – December 2025.

Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex, touching on diverse functions including mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction. This multifacetedness has led to its study being described as "like the fable of the blind men and the elephant".

5-HT receptors, 5-hydroxytryptamine receptors, or serotonin receptors, are a group of G protein-coupled receptor and ligand-gated ion channels found in the central and peripheral nervous systems. They mediate both excitatory and inhibitory neurotransmission. The serotonin receptors are activated by the neurotransmitter serotonin, which acts as their natural ligand.
Neuromodulation is the physiological process by which a given neuron uses one or more chemicals to regulate diverse populations of neurons. Neuromodulators typically bind to metabotropic, G-protein coupled receptors (GPCRs) to initiate a second messenger signaling cascade that induces a broad, long-lasting signal. This modulation can last for hundreds of milliseconds to several minutes. Some of the effects of neuromodulators include: altering intrinsic firing activity, increasing or decreasing voltage-dependent currents, altering synaptic efficacy, increasing bursting activity and reconfigurating synaptic connectivity.

The 5-HT2A receptor is a subtype of the 5-HT2 receptor that belongs to the serotonin receptor family and is a G protein-coupled receptor (GPCR). The 5-HT2A receptor is a cell surface receptor, but has several intracellular locations.

A serotonin receptor agonist is an agonist of one or more serotonin receptors. They activate serotonin receptors in a manner similar to that of serotonin, a neurotransmitter and hormone and the endogenous ligand of the serotonin receptors.

meta-Chlorophenylpiperazine (mCPP) is a psychoactive drug of the phenylpiperazine class. It was initially developed in the late-1970s and used in scientific research before being sold as a designer drug in the mid-2000s. It has been detected in pills touted as legal alternatives to illicit stimulants in New Zealand and pills sold as "ecstasy" in Europe and the United States.
The 5-HT2C receptor is a subtype of 5-HT2 receptor that binds the endogenous neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, it is a G protein-coupled receptor (GPCR) that is coupled to Gq/G11 and mediates excitatory neurotransmission. HTR2C denotes the human gene encoding for the receptor, that in humans is located at the X chromosome. As males have one copy of the gene and in females one of the two copies of the gene is repressed, polymorphisms at this receptor can affect the two sexes to differing extent.
5-Hydroxytryptamine receptor 2B (5-HT2B) also known as serotonin receptor 2B is a protein that in humans is encoded by the HTR2B gene. 5-HT2B is a member of the 5-HT2 receptor family that binds the neurotransmitter serotonin (5-hydroxytryptamine, 5-HT). Like all 5-HT2 receptors, the 5-HT2A receptor is Gq/G11-protein coupled, leading to downstream activation of phospholipase C.
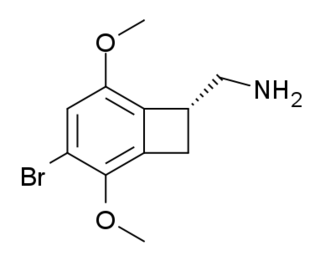
TCB-2 is a hallucinogen discovered in 2006 by Thomas McLean working in the lab of David Nichols at Purdue University. It is a conformationally-restricted derivative of the phenethylamine 2C-B, also a hallucinogen, and acts as a potent agonist for the 5-HT2A and 5-HT2C receptors with a Ki of 0.26 nM at the human 5-HT2A receptor. In drug-substitution experiments in rats, TCB-2 was found to be of similar potency to both LSD and Bromo-DragonFLY, ranking it among the most potent phenethylamine hallucinogens yet discovered. This high potency and selectivity has made TCB-2 useful for distinguishing 5-HT2A mediated responses from those produced by other similar receptors. TCB-2 has similar but not identical effects in animals to related phenethylamine hallucinogens such as DOI, and has been used for studying how the function of the 5-HT2A receptor differs from that of other serotonin receptors in a number of animal models, such as studies of cocaine addiction and neuropathic pain.

Kevin J. Tracey, a neurosurgeon and inventor, is the president and CEO of the Feinstein Institute for Medical Research, professor of neurosurgery and molecular medicine at the Zucker School of Medicine, and president of the Elmezzi Graduate School of Molecular Medicine in Manhasset, New York. The Public Library of Science Magazine, PLOS Biology, recognized Tracey in 2019 as one of the most cited researchers in the world.
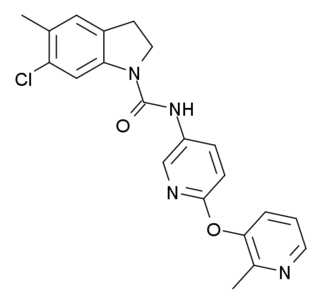
SB-242084 is a psychoactive drug and research chemical which acts as a selective antagonist for the 5HT2C receptor. It has anxiolytic effects, and enhances dopamine signalling in the limbic system, as well as having complex effects on the dopamine release produced by cocaine, increasing it in some brain regions but reducing it in others. It has been shown to increase the effectiveness of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants, and may also reduce their side effects. In animal studies, SB-242084 produced stimulant-type activity and reinforcing effects, somewhat similar to but much weaker than cocaine or amphetamines.

PNU-22394 is a drug which acts as an agonist at serotonin 5-HT2 receptors, with strongest binding affinity for 5-HT2A and 5-HT2C and slightly weaker at 5-HT2B, although it is only a full agonist at 5-HT2C, but partial agonist at 5-HT2A and 5-HT2B. It has anorectic effects in both animal studies and human trials, along with "Pro-Cognitive Properties", although it has never been developed for medical use.

Indorenate (TR-3369), is a tryptamine derivative which acts as an agonist at the 5-HT1A, 5-HT1B and 5-HT2C serotonin receptors. It has anxiolytic, antihypertensive and anorectic effects, predominantly through action at 5-HT1A, but with some contribution from the 5-HT1B and 5-HT2C subtypes, and possibly some other non-serotonergic targets also.

SB-206553 is a drug which acts as a mixed antagonist for the 5-HT2B and 5-HT2C serotonin receptors. It has anxiolytic properties in animal studies and interacts with a range of other drugs. It has also been shown to act as a positive allosteric modulator of α7 nicotinic acetylcholine receptors. Modified derivatives of SB-206553 have been used to probe the structure of the 5-HT2B receptor.

6-Chloro-5-ethoxy-N-(pyridin-2-yl)indoline-1-carboxamide (CEPC) is a drug which acts as a potent and selective antagonist for the serotonin 5-HT2C receptor. In animal studies it was found to potentiate the conditioned place preference induced by low-dose amphetamine, demonstrating that 5-HT2C-mediated disinhibition of dopamine release can cause interactions with dopaminergic drugs.
5-HT2C receptor agonists are a class of drugs that activate 5-HT2C receptors. They have been investigated for the treatment of a number of conditions including obesity, psychiatric disorders, sexual dysfunction and urinary incontinence.

25CN-NBOH is a compound indirectly derived from the phenethylamine series of hallucinogens, which was discovered in 2014 at the University of Copenhagen. This compound is notable as one of the most selective agonist ligands for the 5-HT2A receptor yet discovered, with a pKi of 8.88 at the human 5-HT2A receptor and with 100x selectivity for 5-HT2A over 5-HT2C, and 46x selectivity for 5-HT2A over 5-HT2B. A tritiated version of 25CN-NBOH has also been accessed and used for more detailed investigations of the binding to 5-HT2 receptors and autoradiography.

DMBMPP, or 2-(2,5-dimethoxy-4-bromobenzyl)-6-(2-methoxyphenyl)piperidine, is a 2-benzylpiperidine analog of the hallucinogenic N-benzylphenethylamine 25B-NBOMe and was discovered in 2011 by Jose Juncosa in the group of David E. Nichols at Purdue University. DMBMPP differs from 25B-NBOMe by incorporating the amine within a piperidine ring, making for a more rigid molecular structure than that of the open-chain 25B-NBOMe. The presence of the piperidine ring introduces two stereocenters, thus, four stereoisomers of this compound can be made.

WAY-163,909 is a drug which acts as a potent and reasonably selective agonist for the serotonin 5-HT2C receptor. It has antipsychotic-like effects in animal models, and has been used to study the role of the 5-HT2C receptor subtype in the action of addictive drugs such as nicotine and methamphetamine.

Tenilapine is an atypical antipsychotic which has never been marketed in the US.