Diagnosis
| | This section is empty. You can help by adding to it. (November 2024) |
Glucocorticoid deficiency is a condition where the body doesn't produce enough glucocorticoid hormones.
Symptoms of glucocorticoid deficiency (having not enough hormones that are classified as glucocorticoids, and mostly consisting of cortisol) vary depending on the underlying cause and severity—common signs and symptoms may include fatigue, weakness, weight loss, decreased appetite, low blood pressure, salt cravings, dizziness upon standing (orthostatic hypotension), muscle aches and pains, joint pain or stiffness, nausea or vomiting. In severe cases, individuals may experience abdominal pain, confusion or delirium. [1] [2]
If left untreated or inadequately treated, glucocorticoid deficiency can lead to severe complications such as adrenal crisis (a life-threatening condition characterized by extreme fatigue, dehydration, and electrolyte imbalances), low blood sugar (hypoglycemia), and recurrent infections due to impaired immune function. [2] [1]
Glucocorticoid deficiency can be caused by inherited genetic disorders that affect the production of cortisol in the adrenal glands, such as familial glucocorticoid deficiency (FGD). [3] FGD is a group of monogenic recessive disorders caused by disease-causing variants in genes involved in cortisol biosynthesis. [4] FGD is categorized into type 1, also called Glucocorticoid deficiency 1, where genes associated with ACTH have disease-causing variants, type 2, where ACTH receptors are normal, [5] and there are other types: 3, 4 and 5, associated with various causes. Glucocorticoid deficiency can also be acquired after birth due to damage or dysfunction of the hypothalamus-pituitary-adrenal axis (HPA axis), which regulates cortisol production. [4] Common causes of acquired glucocorticoid deficiency include autoimmune conditions affecting the HPA axis (such as autoimmune adrenalitis) and infections, such as tuberculosis, that affect the adrenal glands directly. [2]
Risk factors for developing glucocorticoid deficiency include having a family history of inherited genetic disorders affecting cortisol biosynthesis by the body; having autoimmune conditions such as Addison's disease; having infections that specifically target the adrenal glands; undergoing surgery involving removal of the pituitary gland or parts of it. [1]
Glucocorticoid deficiency can be triggered by stressors such as illness/infection/surgery/trauma, which increase demand for cortisol production from the adrenals. [4] [6] In individuals with already compromised HPA axis function or inadequate cortisol reserve, this increased demand for glucocorticoid hormones cannot be met, leading to acute exacerbation of glucocorticoid insufficiency/adrenal crisis. [4]
| | This section is empty. You can help by adding to it. (November 2024) |
There are no specific measures known for preventing primary forms of glucocorticoid deficiency unless there is an identified genetic mutation causing it. For secondary forms, prevention includes prompt treatment, i.e., addressing underlying causes such as infection and autoimmune diseases. [1] [4]
The treatment of glucocorticoid deficiency involves replacing the deficient cortisol through exogenous glucocorticoid therapy. This typically includes oral administration of synthetic glucocorticoids such as hydrocortisone or prednisone, which aim to mimic the normal physiological secretion of cortisol. The dosage and timing of medication may vary depending on individual needs and circumstances. [1] [7] [4]
With appropriate treatment, most individuals with glucocorticoid deficiency can lead normal lives and have a good prognosis. [4] However, it is important to closely monitor hormone levels and adjust medication accordingly to prevent complications such as adrenal crisis or over-replacement side effects. [1]
The exact incidence and prevalence of glucocorticoid deficiency are not well established due to its heterogeneity in causes and clinical presentation. [4] Familial Glucocorticoid Deficiency is considered rare but more common than previously thought due to underdiagnosis and lack of awareness about the condition. [1] [3]

The adrenal glands are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis.
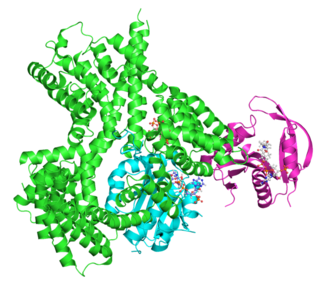
Adrenocorticotropic hormone is a polypeptide tropic hormone produced by and secreted by the anterior pituitary gland. It is also used as a medication and diagnostic agent. ACTH is an important component of the hypothalamic-pituitary-adrenal axis and is often produced in response to biological stress. Its principal effects are increased production and release of cortisol and androgens by the zona fasiculata and zona reticularis, respectively. ACTH is also related to the circadian rhythm in many organisms.

Cushing's syndrome is a collection of signs and symptoms due to prolonged exposure to glucocorticoids such as cortisol. Signs and symptoms may include high blood pressure, abdominal obesity but with thin arms and legs, reddish stretch marks, a round red face due to facial plethora, a fat lump between the shoulders, weak muscles, weak bones, acne, and fragile skin that heals poorly. Women may have more hair and irregular menstruation or loss of menses, with the exact mechanisms of why still unknown. Occasionally there may be changes in mood, headaches, and a chronic feeling of tiredness.

The hypothalamic–pituitary–adrenal axis is a complex set of direct influences and feedback interactions among three components: the hypothalamus, the pituitary gland, and the adrenal glands. These organs and their interactions constitute the HPS axis.

Cortisol is a steroid hormone in the glucocorticoid class of hormones and a stress hormone. When used as medication, it is known as hydrocortisone.

Addison's disease, also known as primary adrenal insufficiency, is a rare long-term endocrine disorder characterized by inadequate production of the steroid hormones cortisol and aldosterone by the two outer layers of the cells of the adrenal glands, causing adrenal insufficiency. Symptoms generally come on slowly and insidiously and may include abdominal pain and gastrointestinal abnormalities, weakness, and weight loss. Darkening of the skin in certain areas may also occur. Under certain circumstances, an adrenal crisis may occur with low blood pressure, vomiting, lower back pain, and loss of consciousness. Mood changes may also occur. Rapid onset of symptoms indicates acute adrenal failure, which is a clinical emergency. An adrenal crisis can be triggered by stress, such as from an injury, surgery, or infection.
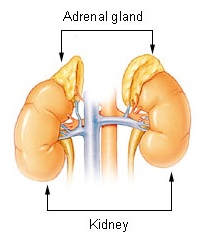
Adrenal insufficiency is a condition in which the adrenal glands do not produce adequate amounts of steroid hormones. The adrenal glands—also referred to as the adrenal cortex—normally secrete glucocorticoids, mineralocorticoids, and androgens. These hormones are important in regulating blood pressure, electrolytes, and metabolism as a whole. Deficiency of these hormones leads to symptoms ranging from abdominal pain, vomiting, muscle weakness and fatigue, low blood pressure, depression, mood and personality changes to organ failure and shock. Adrenal crisis may occur if a person having adrenal insufficiency experiences stresses, such as an accident, injury, surgery, or severe infection; this is a life-threatening medical condition resulting from severe deficiency of cortisol in the body. Death may quickly follow.
Corticotropic cells, are basophilic cells in the anterior pituitary that produce pro-opiomelanocortin (POMC) which undergoes cleavage to adrenocorticotropin (ACTH), β-lipotropin (β-LPH), and melanocyte-stimulating hormone (MSH). These cells are stimulated by corticotropin releasing hormone (CRH) and make up 15–20% of the cells in the anterior pituitary. The release of ACTH from the corticotropic cells is controlled by CRH, which is formed in the cell bodies of parvocellular neurosecretory cells within the paraventricular nucleus of the hypothalamus and passes to the corticotropes in the anterior pituitary via the hypophyseal portal system. Adrenocorticotropin hormone stimulates the adrenal cortex to release glucocorticoids and plays an important role in the stress response.

Lipoid congenital adrenal hyperplasia is an endocrine disorder that is an uncommon and potentially lethal form of congenital adrenal hyperplasia (CAH). It arises from defects in the earliest stages of steroid hormone synthesis: the transport of cholesterol into the mitochondria and the conversion of cholesterol to pregnenolone—the first step in the synthesis of all steroid hormones. Lipoid CAH causes mineralocorticoid deficiency in affected infants and children. Male infants are severely undervirilized causing their external genitalia to look feminine. The adrenals are large and filled with lipid globules derived from cholesterol.
Congenital adrenal hyperplasia due to 17α-hydroxylase deficiency is an uncommon form of congenital adrenal hyperplasia (CAH) resulting from a mutation in the gene CYP17A1, which produces the enzyme 17α-hydroxylase. It causes decreased synthesis of cortisol and sex hormones, with resulting increase in mineralocorticoid production. Thus, common symptoms include mild cortisol deficiency, ambiguous genitalia in men or amenorrhea at puberty in women, and hypokalemic hypertension. However, partial (incomplete) deficiency often has inconsistent symptoms between patients, and affected women may be asymptomatic except for infertility.

Hypopituitarism is the decreased (hypo) secretion of one or more of the eight hormones normally produced by the pituitary gland at the base of the brain. If there is decreased secretion of one specific pituitary hormone, the condition is known as selective hypopituitarism. If there is decreased secretion of most or all pituitary hormones, the term panhypopituitarism is used.

Adrenocorticotropic hormone deficiency is a rare disorder characterized by secondary adrenal insufficiency with minimal or no cortisol production and normal pituitary hormone secretion apart from ACTH. ACTH deficiency may be congenital or acquired, and its symptoms are clinically similar to those of glucocorticoid deficiency. Symptoms consist of weight loss, diminished appetite, muscle weakness, nausea, vomiting, and hypotension. Low blood sugar and hyponatremia are possible; however, blood potassium levels typically remain normal because affected patients are deficient in glucocorticoids rather than mineralocorticoids because of their intact renin-angiotensin-aldosterone system. ACTH may be undetectable in blood tests, and cortisol is abnormally low. Glucocorticoid replacement therapy is required. With the exception of stressful situations, some patients with mild or nearly asymptomatic disease may not require glucocorticoid replacement therapy. As of 2008 about two hundred cases have been described in the literature.

Glucocorticoid deficiency 1 is an adrenocortical failure characterized by low levels of plasma cortisol produced by the adrenal gland despite high levels of plasma ACTH. This is an inherited disorder with several different causes which define the type.
Stress hormones are secreted by endocrine glands to modify one's internal environment during the times of stress. By performing various functions such as mobilizing energy sources, increasing heart rate, and downregulating metabolic processes which are not immediately necessary, stress hormones promote the survival of the organism. The secretions of some hormones are also downplayed during stress. Stress hormones include, but are not limited to:

The adrenocorticotropic hormone receptor or ACTH receptor also known as the melanocortin receptor 2 or MC2 receptor is a type of melanocortin receptor (type 2) which is specific for ACTH. A G protein–coupled receptor located on the external cell plasma membrane, it is coupled to Gαs and upregulates levels of cAMP by activating adenylyl cyclase. The ACTH receptor plays a role in immune function and glucose metabolism.
The ACTH test is a medical test usually requested and interpreted by endocrinologists to assess the functioning of the adrenal glands' stress response by measuring the adrenal response to adrenocorticotropic hormone or another corticotropic agent such as tetracosactide or alsactide (Synchrodyn). ACTH is a hormone produced in the anterior pituitary gland that stimulates the adrenal glands to release cortisol, dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and aldosterone.

Adrenal crisis, also known as Addisonian crisis or acute adrenal insufficiency, is a life-threatening complication of adrenal insufficiency. Hypotension, and hypovolemic shock, are the main symptoms of an adrenal crisis. Other symptoms include weakness, anorexia, nausea, vomiting, fever, fatigue, abnormal electrolytes, confusion, and coma. Laboratory testing may detect low sodium, high potassium, high lymphocyte count, high eosinophils, low blood sugar, and rarely high calcium.

Adrenal gland disorders are conditions that interfere with the normal functioning of the adrenal glands. Your body produces too much or too little of one or more hormones when you have an adrenal gland dysfunction. The type of issue you have and the degree to which it affects your body's hormone levels determine the symptoms.

Melanocortin 2 receptor accessory protein is a transmembrane accessory protein that in humans is encoded by the MRAP gene located in chromosome 21q22.11. Alternate splicing of the MRAP mRNA generates two functionally isoforms MRAP-α and MRAP-β.

Generalized glucocorticoid resistance or Chrousos syndrome is a rare genetic disorder that can run in families or be sporadic. It is characterized by partial or generalized target-tissue insensitivity to glucocorticoids.