The Transporter Classification Database is an International Union of Biochemistry and Molecular Biology (IUBMB)-approved classification system for membrane transport proteins, including ion channels.
The sodium/phosphate cotransporter is a member of the phosphate:Na+ symporter (PNaS) family within the TOG Superfamily of transport proteins as specified in the Transporter Classification Database (TCDB).
A neurotransmitter sodium symporter (NSS) (TC# 2.A.22) is type of neurotransmitter transporter that catalyzes the uptake of a variety of neurotransmitters, amino acids, osmolytes and related nitrogenous substances by a solute:Na+ symport mechanism. The NSS family is a member of the APC superfamily. Its constituents have been found in bacteria, archaea and eukaryotes.
In enzymology, a polyphosphate kinase, or polyphosphate polymerase, is an enzyme that catalyzes the formation of polyphosphate from ATP, with chain lengths of up to a thousand or more orthophosphate moieties.
The Nucleobase:Cation Symporter-1 (NCS1) Family (TC# 2.A.39) consists of over 1000 currently sequenced proteins derived from Gram-negative and Gram-positive bacteria, archaea, fungi and plants. These proteins function as transporters for nucleobases including purines and pyrimidines. Members of this family possess twelve transmembrane α-helical spanners (TMSs). At least some of them have been shown to function in uptake by substrate:H+ symport mechanism.

Members of the Solute:Sodium Symporter (SSS) Family (TC# 2.A.21) catalyze solute:Na+ symport. The SSS family is within the APC Superfamily. The solutes transported may be sugars, amino acids, organo cations such as choline, nucleosides, inositols, vitamins, urea or anions, depending on the system. Members of the SSS family have been identified in bacteria, archaea and eukaryotes. Almost all functionally well-characterized members normally catalyze solute uptake via Na+ symport.
Proteins of the Proton-dependent Oligopeptide Transporter (POT) Family are found in animals, plants, yeast, archaea and both Gram-negative and Gram-positive bacteria, and are part of the major facilitator superfamily. The transport of peptides into cells is a well-documented biological phenomenon which is accomplished by specific, energy-dependent transporters found in a number of organisms as diverse as bacteria and humans. The proton-dependent oligopeptide transporter (PTR) family of proteins is distinct from the ABC-type peptide transporters and was uncovered by sequence analyses of a number of recently discovered peptide transport proteins. These proteins that seem to be mainly involved in the intake of small peptides with the concomitant uptake of a proton.
The Nucleobase cation symporter-2 (NCS2) family, also called the Nucleobase ascorbate transporter (NAT) family, consists of over 1000 sequenced proteins derived from gram-negative and gram-positive bacteria, archaea, fungi, plants and animals. The NCS2/NAT family is a member of the APC Superfamily of secondary carriers. Of the five known families of transporters that act on nucleobases, NCS2/NAT is the only one that is most widespread. Many functionally characterized members are specific for nucleobases including both purines and pyrimidines, but others are purine-specific. However, two closely related rat/human members of the family, SVCT1 and SVCT2, localized to different tissues of the body, co-transport L-ascorbate (vitamin C) and Na+ with a high degree of specificity and high affinity for the vitamin. Clustering of NCS2/NAT family members on the phylogenetic tree is complex, with bacterial proteins and eukaryotic proteins each falling into at least three distinct clusters. The plant and animal proteins cluster loosely together, but the fungal proteins branch from one of the three bacterial clusters forming a tighter grouping. E. coli possesses four distantly related paralogous members of the NCS2 family.
The amino acid-polyamine-organocation (APC) superfamily is the second largest superfamily of secondary carrier proteins currently known, and it contains several Solute carriers. Originally, the APC superfamily consisted of subfamilies under the transporter classification number. This superfamily has since been expanded to include eighteen different families.
The cation-chloride cotransporter (CCC) family is part of the APC superfamily of secondary carriers. Members of the CCC family are found in animals, plants, fungi and bacteria. Most characterized CCC family proteins are from higher eukaryotes, but one has been partially characterized from Nicotiana tabacum, and homologous ORFs have been sequenced from Caenorhabditis elegans (worm), Saccharomyces cerevisiae (yeast) and Synechococcus sp.. The latter proteins are of unknown function. These proteins show sequence similarity to members of the APC family. CCC family proteins are usually large, and possess 12 putative transmembrane spanners (TMSs) flanked by large N-terminal and C-terminal hydrophilic domains.
The anion exchanger family is a member of the large APC superfamily of secondary carriers. Members of the AE family are generally responsible for the transport of anions across cellular barriers, although their functions may vary. All of them exchange bicarbonate. Characterized protein members of the AE family are found in plants, animals, insects and yeast. Uncharacterized AE homologues may be present in bacteria. Animal AE proteins consist of homodimeric complexes of integral membrane proteins that vary in size from about 900 amino acyl residues to about 1250 residues. Their N-terminal hydrophilic domains may interact with cytoskeletal proteins and therefore play a cell structural role. Some of the currently characterized members of the AE family can be found in the Transporter Classification Database.
The SWEET family, also known as the PQ-loop, Saliva or MtN3 family, is a family of sugar transporters and a member of the TOG superfamily. The proteins of the SWEET family have been found in plants, animals, protozoans, and bacteria. Eukaryotic family members have 7 transmembrane segments (TMSs) in a 3+1+3 repeat arrangement.

Natural resistance-associated macrophage proteins (Nramps), also known as metal ion (Mn2+-iron) transporters (TC# 2.A.55), are a family of metal transport proteins found throughout all domains of life. Taking on an eleven-helix LeuT fold, the Nramp family is a member of the large APC Superfamily of secondary carriers. They transport a variety of transition metals such as manganese, cadmium, and manganese using an alternating access mechanism characteristic of secondary transporters.
The potassium (K+) uptake permease (KUP) family (TC# 2.A.72) is a member of the APC superfamily of secondary carriers. Proteins of the KUP/HAK/KT family include the KUP (TrkD) protein of E. coli and homologues in both Gram-positive and Gram-negative bacteria. High affinity (20 μM) K+ uptake systems (Hak1, TC# 2.A.72.2.1) of the yeast Debaryomyces occidentalis as well as the fungus, Neurospora crassa, and several homologues in plants have been characterized. Arabidopsis thaliana and other plants possess multiple KUP family paralogues. While many plant proteins cluster tightly together, the Hak1 proteins from yeast as well as the two Gram-positive and Gram-negative bacterial proteins are distantly related on the phylogenetic tree for the KUP family. All currently classified members of the KUP family can be found in the Transporter Classification Database.
The Ca2+:cation antiporter (CaCA) family (TC# 2.A.19) is a member of the cation diffusion facilitator (CDF) superfamily. This family should not be confused with the Ca2+:H+ Antiporter-2 (CaCA2) Family (TC# 2.A.106) which belongs to the Lysine Exporter (LysE) Superfamily. Proteins of the CaCA family are found ubiquitously, having been identified in animals, plants, yeast, archaea and divergent bacteria. Members of this family facilitate the antiport of calcium ion with another cation.
The multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) flippase superfamily is a group of integral membrane protein families. The MOP flippase superfamily includes twelve distantly related families, six for which functional data are available:
- One ubiquitous family (MATE) specific for drugs - (TC# 2.A.66.1) The Multi Antimicrobial Extrusion (MATE) Family
- One (PST) specific for polysaccharides and/or their lipid-linked precursors in prokaryotes - (TC# 2.A.66.2) The Polysaccharide Transport (PST) Family
- One (OLF) specific for lipid-linked oligosaccharide precursors of glycoproteins in eukaryotes - (TC# 2.A.66.3) The Oligosaccharidyl-lipid Flippase (OLF) Family
- One (MVF) lipid-peptidoglycan precursor flippase involved in cell wall biosynthesis - (TC# 2.A.66.4) The Mouse Virulence Factor (MVF) Family
- One (AgnG) which includes a single functionally characterized member that extrudes the antibiotic, Agrocin 84 - (TC# 2.A.66.5) The Agrocin 84 Antibiotic Exporter (AgnG) Family
- And finally, one (Ank) that shuttles inorganic pyrophosphate (PPi) - (TC# 2.A.66.9) The Progressive Ankylosis (Ank) Family
Divalent anion:Na+ symporters were found in bacteria, archaea, plant chloroplasts and animals.
The arsenical resistance-3 (ACR3) family is a member of the BART superfamily. Based on operon analyses, ARC3 homologues may function either as secondary carriers or as primary active transporters, similarly to the ArsB and ArsAB families. In the latter case ATP hydrolysis again energizes transport. ARC3 homologues transport the same anions as ArsA/AB homologues, though ArsB homologues are members of the IT Superfamily and homologues of the ARC3 family are within the BART Superfamily suggesting they may not be evolutionarily related.
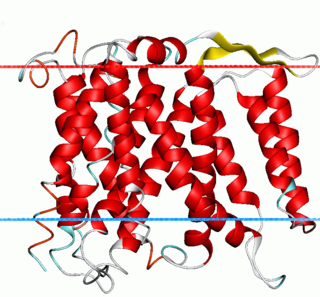
The Monovalent Cation:Proton Antiporter-1 (CPA1) Family (TC# 2.A.36) is a large family of proteins derived from Gram-positive and Gram-negative bacteria, blue-green bacteria, archaea, yeast, plants and animals. The CPA1 family belongs to the VIC superfamily. Transporters from eukaryotes have been functionally characterized to catalyze Na+:H+ exchange. Their primary physiological functions are thought to be in (1) cytoplasmic pH regulation, extruding the H+ generated during metabolism, and (2) salt tolerance (in plants), due to Na+ uptake into vacuoles. Bacterial homologues have also been found to facilitate Na+:H+ antiport, but some also catalyze Li+:H+ antiport or Ca2+:H+ antiport under certain conditions.
The Malonate:Na+ Symporter (MSS) Family (TC# 2.A.70) is a group of transport proteins belonging to the CPA superfamily. These proteins are composites with constituents ranging in size from 129 to 255 amino acyl residues (aas) and exhibiting 4 to 7 transmembrane segments (TMSs). A representative list of proteins belonging to the MSS family can be found in the Transporter Classification Database.


