Glyoxylate and dicarboxylate metabolism describes a variety of reactions involving glyoxylate or dicarboxylates. Glyoxylate is the conjugate base of glyoxylic acid, and within a buffered environment of known pH such as the cell cytoplasm these terms can be used almost interchangeably, as the gain or loss of a hydrogen ion is all that distinguishes them, and this can occur in the aqueous environment at any time. Likewise dicarboxylates are the conjugate bases of dicarboxylic acids, a general class of organic compounds containing two carboxylic acid groups, such as oxalic acid or succinic acid.

Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.

In chemistry, pH is a scale used to specify how acidic or basic a water-based solution is. Acidic solutions have a lower pH, while basic solutions have a higher pH. At room temperature (25 °C), pure water is neither acidic nor basic and has a pH of 7.

In cell biology, the cytoplasm is all of the material within a cell, enclosed by the cell membrane, except for the cell nucleus. The material inside the nucleus and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are cytosol – a gel-like substance, the organelles – the cell's internal sub-structures, and various cytoplasmic inclusions. The cytoplasm is about 80% water and usually colorless.
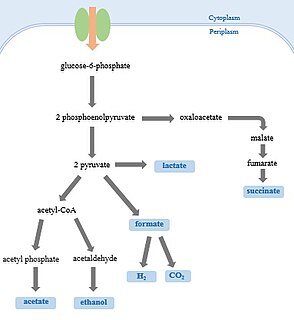
A compact graphical description of major biochemical reactions involved can be found at KEGG [1] This provides information on the relevant enzymes and details the relationship with several other metabolic processes: glycine, serine, and threonine metabolism which provides hydroxypyruvate and glyoxylate, purine metabolism which provides glyoxylate, pyruvate metabolism which provides (S)-malate and formate, carbon fixation which consumes 3-phospho-D-glycerate and provides D-ribulose 1,5-P2, ascorbate and aldarate metabolism which shares tartronate-semialdehyde, nitrogen metabolism which shares formate, pyruvate metabolism and the citrate cycle which share oxaloacetate, and vitamin B6 metabolism which consumes glycolaldehyde.

KEGG is a collection of databases dealing with genomes, biological pathways, diseases, drugs, and chemical substances. KEGG is utilized for bioinformatics research and education, including data analysis in genomics, metagenomics, metabolomics and other omics studies, modeling and simulation in systems biology, and translational research in drug development.

Enzymes are macromolecular biological catalysts. Enzymes accelerate chemical reactions. The molecules upon which enzymes may act are called substrates and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called enzymology and a new field of pseudoenzyme analysis has recently grown up, recognising that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties.
Purine metabolism refers to the metabolic pathways to synthesize and break down purines that are present in many organisms.
The glyoxylate cycle describes an important subset of these reactions involved in biosynthesis of carbohydrates from fatty acids or two-carbon precursors which enter the system as acetyl-coenzyme A. Its crucial enzymes are isocitrate lyase and malate synthase. However, alternate pathways have been proposed in organisms lacking isocitrate lyase. [2]
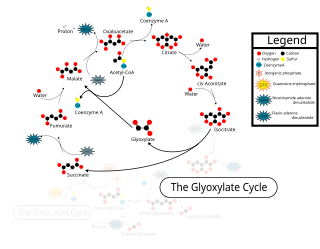
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to utilize simple carbon compounds as a carbon source when complex sources such as glucose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.
In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction