
The citric acid cycle —also known as the Krebs cycle, Szent-Györgyi-Krebs cycle or the TCA cycle (tricarboxylic acid cycle)—is a series of chemical reactions to release stored energy through the oxidation of acetyl-CoA derived from carbohydrates, fats, and proteins. The Krebs cycle is used by organisms that respire (as opposed to organisms that ferment) to generate energy, either by anaerobic respiration or aerobic respiration. In addition, the cycle provides precursors of certain amino acids, as well as the reducing agent NADH, that are used in numerous other reactions. Its central importance to many biochemical pathways suggests that it was one of the earliest components of metabolism. Even though it is branded as a 'cycle', it is not necessary for metabolites to follow only one specific route; at least three alternative segments of the citric acid cycle have been recognized.
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.

Biological carbon fixation or сarbon assimilation is the process by which inorganic carbon is converted to organic compounds by living organisms. The compounds are then used to store energy and as structure for other biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use a process called chemosynthesis in the absence of sunlight.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

Isocitrate dehydrogenase (IDH) (EC 1.1.1.42) and (EC 1.1.1.41) is an enzyme that catalyzes the oxidative decarboxylation of isocitrate, producing alpha-ketoglutarate (α-ketoglutarate) and CO2. This is a two-step process, which involves oxidation of isocitrate (a secondary alcohol) to oxalosuccinate (a ketone), followed by the decarboxylation of the carboxyl group beta to the ketone, forming alpha-ketoglutarate. In humans, IDH exists in three isoforms: IDH3 catalyzes the third step of the citric acid cycle while converting NAD+ to NADH in the mitochondria. The isoforms IDH1 and IDH2 catalyze the same reaction outside the context of the citric acid cycle and use NADP+ as a cofactor instead of NAD+. They localize to the cytosol as well as the mitochondrion and peroxisome.

In the mitochondrion, the matrix is the space within the inner membrane. The word "matrix" stems from the fact that this space is viscous, compared to the relatively aqueous cytoplasm. The mitochondrial matrix contains the mitochondrial DNA, ribosomes, soluble enzymes, small organic molecules, nucleotide cofactors, and inorganic ions.[1] The enzymes in the matrix facilitate reactions responsible for the production of ATP, such as the citric acid cycle, oxidative phosphorylation, oxidation of pyruvate, and the beta oxidation of fatty acids.
Glyoxysomes are specialized peroxisomes found in plants (particularly in the fat storage tissues of germinating seeds) and also in filamentous fungi. Seeds that contain fats and oils include corn, soybean, sunflower, peanut and pumpkin. As in all peroxisomes, in glyoxysomes the fatty acids are oxidized to acetyl-CoA by peroxisomal β-oxidation enzymes. When the fatty acids are oxidized hydrogen peroxide (H2O2) is produced as oxygen (O2) is consumed. Thus the seeds need oxygen to germinate. Besides peroxisomal functions, glyoxysomes possess additionally the key enzymes of the glyoxylate cycle (isocitrate lyase and malate synthase) which accomplish the glyoxylate cycle bypass.
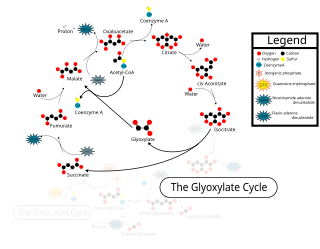
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

In biochemistry, mixed acid fermentation is the metabolic process by which a six-carbon sugar is converted into a complex and variable mixture of acids. It is an anaerobic (non-oxygen-requiring) fermentation reaction that is common in bacteria. It is characteristic for members of the Enterobacteriaceae, a large family of Gram-negative bacteria that includes E. coli.
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; EC 4.1.1.31, PDB ID: 3ZGE) is an enzyme in the family of carboxy-lyases found in plants and some bacteria that catalyzes the addition of bicarbonate (HCO3−) to phosphoenolpyruvate (PEP) to form the four-carbon compound oxaloacetate and inorganic phosphate:
Propionyl-CoA is a coenzyme A derivative of propionic acid. It is composed of a 24 total carbon chain and its production and metabolic fate depend on which organism it is present in. Several different pathways can lead to its production, such as through the catabolism of specific amino acids or the oxidation of odd-chain fatty acids. It later can be broken down by propionyl-CoA carboxylase or through the methylcitrate cycle. In different organisms, however, propionyl-CoA can be sequestered into controlled regions, to alleviate its potential toxicity through accumulation. Genetic deficiencies regarding the production and breakdown of propionyl-CoA also have great clinical and human significance.
In biochemistry, fatty acid synthesis is the creation of fatty acids from acetyl-CoA and NADPH through the action of enzymes called fatty acid synthases. This process takes place in the cytoplasm of the cell. Most of the acetyl-CoA which is converted into fatty acids is derived from carbohydrates via the glycolytic pathway. The glycolytic pathway also provides the glycerol with which three fatty acids can combine to form triglycerides, the final product of the lipogenic process. When only two fatty acids combine with glycerol and the third alcohol group is phosphorylated with a group such as phosphatidylcholine, a phospholipid is formed. Phospholipids form the bulk of the lipid bilayers that make up cell membranes and surrounds the organelles within the cells.

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.

The enzyme methylisocitrate lyase catalyzes the chemical reaction
The enzyme oxalyl-CoA decarboxylase (OXC) (EC 4.1.1.8), primarily produced by the gastrointestinal bacterium Oxalobacter formigenes, catalyzes the chemical reaction

In enzymology, N-acetylglucosamine-6-phosphate deacetylase (EC 3.5.1.25), also known as GlcNAc-6-phosphate deacetylase or NagA, is an enzyme that catalyzes the deacetylation of N-acetylglucosamine-6-phosphate (GlcNAc-6-P) to glucosamine-6-phosphate (GlcN-6-P):
Glyoxylate and dicarboxylate metabolism describes a variety of reactions involving glyoxylate or dicarboxylates. Glyoxylate is the conjugate base of glyoxylic acid, and within a buffered environment of known pH such as the cell cytoplasm these terms can be used almost interchangeably, as the gain or loss of a hydrogen ion is all that distinguishes them, and this can occur in the aqueous environment at any time. Likewise dicarboxylates are the conjugate bases of dicarboxylic acids, a general class of organic compounds containing two carboxylic acid groups, such as oxalic acid or succinic acid.
Isocitrate lyase family is a family of evolutionarily related proteins.
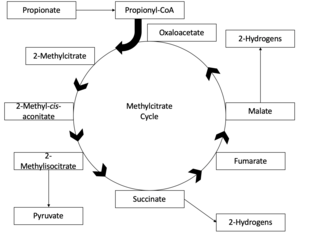
The methylcitrate cycle, or the MCC, is the mechanism by which propionyl-CoA is formed, generated by β-oxidation of odd-chain fatty acids, and broken down to its final products, succinate and pyruvate. The methylcitrate cycle is closely related to both the citric acid cycle and the glyoxylate cycle, in that they share substrates, enzymes and products. The methylcitrate cycle functions overall to detoxify bacteria of toxic propionyl-CoA, and plays an essential role in propionate metabolism in bacteria. Incomplete propionyl-CoA metabolism may lead to the buildup of toxic metabolites in bacteria, and thus the function of the methylcitrate cycle is an important biological process.

















