 | |
 | |
| Names | |
|---|---|
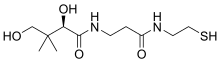
| Systematic IUPAC name (2R)-2,4-Dihydroxy-3,3-dimethyl-N-{3-oxo-3-[(2-sulfanylethyl)amino]propyl}butanamide | |
| Other names Pantetheine | |
| Identifiers | |
| |
3D model (JSmol) | |
| 1714196 R | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.114 |
| EC Number |
|
| KEGG | |
| MeSH | Pantetheine |
PubChem CID | |
| UNII | |
| |
| |
| Properties | |
| C11H22N2O4S | |
| Molar mass | 278.37 g·mol−1 |
| Related compounds | |
Related compounds | Pantethine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Pantetheine is the cysteamine amide analog of pantothenic acid (vitamin B5). The dimer of this compound, pantethine is more commonly known, and is considered to be the most potent form of vitamin B5. Pantetheine is an intermediate in the catabolism of coenzyme A by the body. [1] [2] [3]