In biochemistry, a ligase is an enzyme that can catalyze the joining (ligation) of two molecules by forming a new chemical bond. This is typically via hydrolysis of a small pendant chemical group on one of the molecules, typically resulting in the formation of new C-O, C-S, or C-N bonds. For example, DNA ligase can join two complementary fragments of nucleic acid by forming phosphodiester bonds, and repair single stranded breaks that arise in double stranded DNA during replication.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.
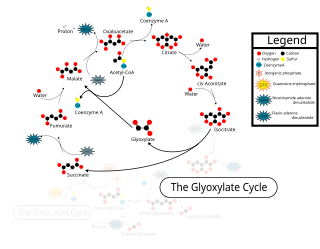
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
In enzymology, a secologanin synthase (EC 1.14.19.62, was wrongly classified as EC 1.3.3.9 in the past) is an enzyme that catalyzes the chemical reaction
In enzymology, a glyoxylate oxidase (EC 1.2.3.5) is an enzyme that catalyzes the chemical reaction

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.
In enzymology, a 2-ethylmalate synthase (EC 2.3.3.6) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxyglutarate synthase (EC 2.3.3.11) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-isopropylmalate synthase (EC 2.3.3.13) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-methylcitrate synthase (EC 2.3.3.5) is an enzyme that catalyzes the chemical reaction
In enzymology, a 3-ethylmalate synthase (EC 2.3.3.7) is an enzyme that catalyzes the chemical reaction
In enzymology, a 6-methylsalicylic-acid synthase (EC 2.3.1.165) is a polyketide synthase that catalyzes the chemical reaction
In enzymology, a citrate (Re)-synthase (EC 2.3.3.3) is an enzyme that catalyzes the chemical reaction
Decylcitrate synthase (EC 2.3.3.2) is an enzyme that catalyzes the chemical reaction in enzymology.
In enzymology, a decylhomocitrate synthase (EC 2.3.3.4) is an enzyme that catalyzes the chemical reaction

In enzymology, a homocitrate synthase (EC 2.3.3.14) is an enzyme that catalyzes the chemical reaction

In molecular biology, hydroxymethylglutaryl-CoA synthase or HMG-CoA synthase EC 2.3.3.10 is an enzyme which catalyzes the reaction in which acetyl-CoA condenses with acetoacetyl-CoA to form 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA). This reaction comprises the second step in the mevalonate-dependent isoprenoid biosynthesis pathway. HMG-CoA is an intermediate in both cholesterol synthesis and ketogenesis. This reaction is overactivated in patients with diabetes mellitus type 1 if left untreated, due to prolonged insulin deficiency and the exhaustion of substrates for gluconeogenesis and the TCA cycle, notably oxaloacetate. This results in shunting of excess acetyl-CoA into the ketone synthesis pathway via HMG-CoA, leading to the development of diabetic ketoacidosis.

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a 2-hydroxy-3-oxoadipate synthase (EC 2.2.1.5) is an enzyme that catalyzes the following chemical reaction:







