Modifications

In 1953, John Ingram, an English dermatologist, added topical anthralin paste to his Goeckerman regimen. This is known as the Ingram method. [5]
Goeckerman therapy is a regimen for treatment of moderate to severe plaque psoriasis using a combination of crude coal tar and artificial ultraviolet radiation. It is a specialized form of light therapy.
First formulated in 1925 by American dermatologist William H. Goeckerman (1884–1954), Goeckerman therapy continues to be used due to its efficacy and safety profile. [1] Individual institutions have modified the Goeckerman regimen and developed their own protocols. Standard therapy includes use of 2–4% crude coal tar in a petroleum base applied daily to the psoriatic plaques. The minimum period of time for tar application is 2-hours, although it has been recognized that greater periods of time produce better results. [2] The patient is then exposed to broad-band ultraviolet B (UVB) radiation, although narrow-band UVB may also be used. [3] Laboratory studies have shown that the combination of coal tar and UV light reduces epidermal DNA synthesis. [4]

In 1953, John Ingram, an English dermatologist, added topical anthralin paste to his Goeckerman regimen. This is known as the Ingram method. [5]
Recent publications have compared Goeckerman therapy with treatment with more expensive biologic agents. [6] [7] Historically, Goeckerman therapy was performed as an inpatient treatment. However, today the treatment can be done with reduced cost as an outpatient. It has been stated by de Miguel et al., that an annual three-week outpatient course of Goeckerman treatment costs $10,000 to 12,000 but repeat treatment may be extended to two years with the use of a $2,000 home UVB treatment lamp. [6] The authors state that biologic therapy costs $22,000 to 59,000 per year.
Goeckerman regimens use crude coal tar, which contains polycyclic aromatic hydrocarbon, a carcinogen. [8] [9] However, Goeckerman therapy is considered safe although use of tar may have the side-effects of contact dermatitis and mild local burning due to tar hypersensitivity. A retrospective study by Stern et al., of 1,373 patients concluded that there was an increase in skin cancers in those receiving repeated Goeckerman treatments compared to the control group. [10] This has been refuted by other authors, including Pittelkow et al., who state there has not been an increase in skin cancers among those treated compared to the general population [11] and Menter and Cran, who felt that the Stern study was too crude to have validity and felt a 10-year prospective study would be needed to confirm safety concerns. [2]
With the increased use of biologic medications in treatment of moderate-to-severe psoriasis there has been a shift away from Goeckerman therapy. A 2007 comparative study of psoriasis treatment found Goeckerman therapy to be more efficacious at 12-weeks than biologics. [12] It has also been successfully used in patients who have failed some biologic therapies. [13]
Coal tar is a thick dark liquid which is a by-product of the production of coke and coal gas from coal. It has both medical and industrial uses. Medicinally it is a topical medication applied to skin to treat psoriasis and seborrheic dermatitis (dandruff). It may be used in combination with ultraviolet light therapy. Industrially it is a railroad tie preservative and used in the surfacing of roads. Coal Tar was listed as a known human carcinogen in the first Report on Carcinogens from the U.S. Federal Government.

Vitiligo is a long-term skin condition characterized by patches of the skin losing their pigment. The patches of skin affected become white and usually have sharp margins. The hair from the skin may also become white. The inside of the mouth and nose may also be involved. Typically both sides of the body are affected. Often the patches begin on areas of skin that are exposed to the sun. It is more noticeable in people with dark skin. Vitiligo may result in psychological stress and those affected are sometimes stigmatized.

Psoriasis is a long-lasting, noncontagious autoimmune disease characterized by raised areas of abnormal skin. These areas are red, or purple on some people with darker skin, dry, itchy, and scaly. Psoriasis varies in severity from small, localized patches to complete body coverage. Injury to the skin can trigger psoriatic skin changes at that spot, which is known as the Koebner phenomenon.

Indoor tanning involves using a device that emits ultraviolet radiation to produce a cosmetic tan. Typically found in tanning salons, gyms, spas, hotels, and sporting facilities, and less often in private residences, the most common device is a horizontal tanning bed, also known as a sunbed or solarium. Vertical devices are known as tanning booths or stand-up sunbeds.

Light therapy—or phototherapy, classically referred to as heliotherapy—consists either of exposure to daylight or some equivalent form of light as a treatment for seasonal affective disorder (SAD), or exposure of the skin to specific wavelengths of light using polychromatic polarised light to treat a skin condition.
Ultraviolet light therapy or ultraviolet phototherapy is a form of treatment for certain skin disorders including atopic skin disorder and vitiligo when used with psoralen to form the PUVA treatment. It consists of irradiation of the patient with the UVA band of ultraviolet light, usually delivered from a fluorescent bulb specially designed to output this frequency of ultraviolet.
PUVA is an ultraviolet light therapy treatment for skin diseases: Eczema, Psoriasis, Graft-versus-host disease, Vitiligo, Mycosis Fungoides, Large Plaque Parapsoriasis and cutaneous T-cell lymphoma using the sensitizing effects of the drug Psoralen. The psoralen is applied or taken orally to sensitize the skin, then the skin is exposed to UVA.

Ammonium bituminosulfonate or ammonium bituminosulphonate is a product of natural origin obtained in the first step by dry distillation of sulfur-rich oil shale. By sulfonation of the resulting oil, and subsequent neutralization with ammonia, Ichthammol results as a viscous, water-soluble substance with a characteristic bitumen-like odor. It is used in medicine as a treatment for different skin diseases, including eczema and psoriasis. Ointments containing 10% or 20% Ichthammol are most common. They are sometimes called "black ointments" or "drawing salves". Ichthammol's dermatological action was promoted by German physician Paul Gerson Unna.

Balneotherapy is a method of treating diseases by bathing, a traditional medicine technique usually practiced at spas. While it is considered distinct from hydrotherapy, there are some overlaps in practice and in underlying principles. Balneotherapy may involve hot or cold water, massage through moving water, relaxation, or stimulation. Many mineral waters at spas are rich in particular minerals such as silica, sulfur, selenium, and radium. Medicinal clays are also widely used, a practice known as 'fangotherapy'.
A TNF inhibitor is a pharmaceutical drug that suppresses the physiologic response to tumor necrosis factor (TNF), which is part of the inflammatory response. TNF is involved in autoimmune and immune-mediated disorders such as rheumatoid arthritis, ankylosing spondylitis, inflammatory bowel disease, psoriasis, hidradenitis suppurativa and refractory asthma, so TNF inhibitors may be used in their treatment. The important side effects of TNF inhibitors include lymphomas, infections, congestive heart failure, demyelinating disease, a lupus-like syndrome, induction of auto-antibodies, injection site reactions, and systemic side effects.
UV-B lamps are lamps that emit a spectrum of ultraviolet light with wavelengths ranging from 290–320 nanometers. This spectrum is also commonly called the biological spectrum due to the human body's sensitivity to light of such a wavelength. UV-B light does not tan the skin very much, compared to the UV-A lamps that are used in tanning beds.

Psoriatic onychodystrophy or psoriatic nails is a nail disease. It is common in those suffering from psoriasis, with reported incidences varying from 10% to 78%. Elderly patients and those with psoriatic arthritis are more likely to have psoriatic nails.
Generalized pustular psoriasis (GPP) is an extremely rare type of psoriasis that can present in a variety of forms. Unlike the most general and common forms of psoriasis, GPP usually covers the entire body and with pus-filled blisters rather than plaques. GPP can present at any age, but is rarer in young children. It can appear with or without previous psoriasis conditions or history, and can reoccur in periodic episodes.

Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis.
Mark G. Lebwohl, M.D., is an American dermatologist and author and the Waldman Professor and Chairman of the Kimberly and Eric J. Waldman Department of and Chairman of the Department of Dermatology at the Mount Sinai Hospital in New York City.
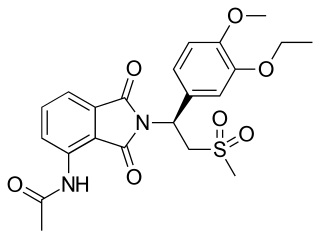
Apremilast, sold under the brand name Otezla among others, is a medication for the treatment of certain types of psoriasis and psoriatic arthritis. It may also be useful for other immune system-related inflammatory diseases. The drug acts as a selective inhibitor of the enzyme phosphodiesterase 4 (PDE4) and inhibits spontaneous production of TNF-alpha from human rheumatoid synovial cells. It is taken by mouth.
Tildrakizumab is a monoclonal antibody designed for the treatment of immunologically mediated inflammatory disorders. It is approved for the treatment of adult patients with moderate-to-severe plaque psoriasis in the United States and the European Union.

Calcipotriol/betamethasone dipropionate, sold under the brand name Taclonex among others, is a fixed-dose combination medication of the synthetic vitamin D3 analog calcipotriol (also known as calcipotriene) and the synthetic corticosteroid betamethasone dipropionate for the treatment of plaque psoriasis. It is used in the form of ointment, topical suspension, gel, aerosol, and foam.
Risankizumab, sold under the brand name Skyrizi, is a humanized monoclonal antibody targeting interleukin 23A (IL-23A). Risankizumab is part of a collaboration between Boehringer Ingelheim and AbbVie. Risankizumab has been approved in the European Union, the United States, and Canada for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy. In Japan, it is approved for treating plaque psoriasis, generalized pustular psoriasis, erythrodermic psoriasis and psoriatic arthritis in adults who have an inadequate response to conventional therapies.
von Zumbusch (acute) generalized pustular psoriasis is the most severe form of generalized pustular psoriasis, and can be associated with life-threatening complications.