
Infrared is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with waves that are just longer than those of red light, so IR is invisible to the human eye. IR is generally understood to include wavelengths from around 750 nm (400 THz) to 1 mm (300 GHz). IR is commonly divided between longer-wavelength thermal IR, emitted from terrestrial sources, and shorter-wavelength IR or near-IR, part of the solar spectrum. Longer IR wavelengths (30–100 μm) are sometimes included as part of the terahertz radiation band. Almost all black-body radiation from objects near room temperature is in the IR band. As a form of electromagnetic radiation, IR carries energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon.
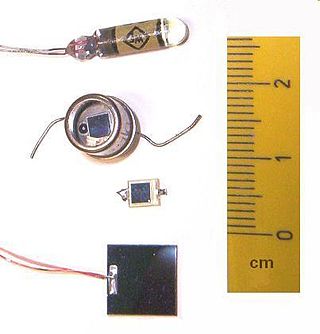
A photodiode is a semiconductor diode sensitive to photon radiation, such as visible light, infrared or ultraviolet radiation, X-rays and gamma rays. It produces an electrical current when it absorbs photons. This can be used for detection and measurement applications, or for the generation of electrical power in solar cells. Photodiodes are used in a wide range of applications throughout the electromagnetic spectrum from visible light photocells to gamma ray spectrometers.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

A thermographic camera is a device that creates an image using infrared (IR) radiation, similar to a normal camera that forms an image using visible light. Instead of the 400–700 nanometre (nm) range of the visible light camera, infrared cameras are sensitive to wavelengths from about 1,000 nm to about 14,000 nm (14 μm). The practice of capturing and analyzing the data they provide is called thermography.

Absorption spectroscopy is spectroscopy that involves techniques that measure the absorption of electromagnetic radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum.

Far infrared (FIR) or long wave refers to a specific range within the infrared spectrum of electromagnetic radiation. It encompasses radiation with wavelengths ranging from 15 μm (micrometers) to 1 mm, which corresponds to a frequency range of approximately 20 THz to 300 GHz. This places far infrared radiation within the CIE IR-B and IR-C bands. The longer wavelengths of the FIR spectrum overlap with a range known as terahertz radiation. Different sources may use different boundaries to define the far infrared range. For instance, astronomers often define it as wavelengths between 25 μm and 350 μm. Infrared photons possess significantly lower energy than photons in the visible light spectrum, with tens to hundreds of times less energy.

Photodetectors, also called photosensors, are sensors of light or other electromagnetic radiation. There are a wide variety of photodetectors which may be classified by mechanism of detection, such as photoelectric or photochemical effects, or by various performance metrics, such as spectral response. Semiconductor-based photodetectors typically use a p–n junction that converts photons into charge. The absorbed photons make electron–hole pairs in the depletion region. Photodiodes and photo transistors are a few examples of photo detectors. Solar cells convert some of the light energy absorbed into electrical energy.

Total organic carbon (TOC) is an analytical parameter representing the concentration of organic carbon in a sample. TOC determinations are made in a variety of application areas. For example, TOC may be used as a non-specific indicator of water quality, or TOC of source rock may be used as one factor in evaluating a petroleum play. For marine surface sediments average TOC content is 0.5% in the deep ocean, and 2% along the eastern margins.

Marcel Jules Edouard Golay was a Swiss mathematician, physicist, and information theorist, who applied mathematics to real-world military and industrial problems. He was born in Neuchâtel, Switzerland.

Hg1−xCdxTe or mercury cadmium telluride is a chemical compound of cadmium telluride (CdTe) and mercury telluride (HgTe) with a tunable bandgap spanning the shortwave infrared to the very long wave infrared regions. The amount of cadmium (Cd) in the alloy can be chosen so as to tune the optical absorption of the material to the desired infrared wavelength. CdTe is a semiconductor with a bandgap of approximately 1.5 electronvolts (eV) at room temperature. HgTe is a semimetal, which means that its bandgap energy is zero. Mixing these two substances allows one to obtain any bandgap between 0 and 1.5 eV.

An infrared detector is a detector that reacts to infrared (IR) radiation. The two main types of detectors are thermal and photonic (photodetectors).
Lead selenide (PbSe), or lead(II) selenide, a selenide of lead, is a semiconductor material. It forms cubic crystals of the NaCl structure; it has a direct bandgap of 0.27 eV at room temperature. A grey solid, it is used for manufacture of infrared detectors for thermal imaging. The mineral clausthalite is a naturally occurring lead selenide.
Chemical imaging is the analytical capability to create a visual image of components distribution from simultaneous measurement of spectra and spatial, time information. Hyperspectral imaging measures contiguous spectral bands, as opposed to multispectral imaging which measures spaced spectral bands.
A gas detector is a device that detects the presence of gases in an area, often as part of a safety system. A gas detector can sound an alarm to operators in the area where the leak is occurring, giving them the opportunity to leave. This type of device is important because there are many gases that can be harmful to organic life, such as humans or animals.
Photothermal microspectroscopy (PTMS), alternatively known as photothermal temperature fluctuation (PTTF), is derived from two parent instrumental techniques: infrared spectroscopy and atomic force microscopy (AFM). In one particular type of AFM, known as scanning thermal microscopy (SThM), the imaging probe is a sub-miniature temperature sensor, which may be a thermocouple or a resistance thermometer. This same type of detector is employed in a PTMS instrument, enabling it to provide AFM/SThM images: However, the chief additional use of PTMS is to yield infrared spectra from sample regions below a micrometer, as outlined below.

The ability to sense infrared thermal radiation evolved independently in two different groups of snakes, one consisting of the families Boidae (boas) and Pythonidae (pythons), the other of the family Crotalinae. What is commonly called a pit organ allows these animals to essentially "see" radiant heat at wavelengths between 5 and 30 μm. The more advanced infrared sense of pit vipers allows these animals to strike prey accurately even in the absence of light, and detect warm objects from several meters away. It was previously thought that the organs evolved primarily as prey detectors, but recent evidence suggests that it may also be used in thermoregulation and predator detection, making it a more general-purpose sensory organ than was supposed.
A flame detector is a sensor designed to detect and respond to the presence of a flame or fire, allowing flame detection. Responses to a detected flame depend on the installation, but can include sounding an alarm, deactivating a fuel line, and activating a fire suppression system. When used in applications such as industrial furnaces, their role is to provide confirmation that the furnace is working properly; it can be used to turn off the ignition system though in many cases they take no direct action beyond notifying the operator or control system. A flame detector can often respond faster and more accurately than a smoke or heat detector due to the mechanisms it uses to detect the flame.

An infrared gas analyzer measures trace gases by determining the absorption of an emitted infrared light source through a certain air sample. Trace gases found in the Earth's atmosphere become excited under specific wavelengths found in the infrared range. The concept behind the technology can be understood as testing how much of the light is absorbed by the air. Different molecules in the air absorb different frequencies of light. Air with much of a certain gas will absorb more of a certain frequency, allowing the sensor to report a high concentration of the corresponding molecule.

A transition-edge sensor (TES) is a type of cryogenic energy sensor or cryogenic particle detector that exploits the strongly temperature-dependent resistance of the superconducting phase transition.

X-ray detectors are devices used to measure the flux, spatial distribution, spectrum, and/or other properties of X-rays.















