In toxicology, the median lethal dose, LD50 (abbreviation for "lethal dose, 50%"), LC50 (lethal concentration, 50%) or LCt50 is a toxic unit that measures the lethal dose of a given substance. The value of LD50 for a substance is the dose required to kill half the members of a tested population after a specified test duration. LD50 figures are frequently used as a general indicator of a substance's acute toxicity. A lower LD50 is indicative of higher toxicity.

Toxicology is a scientific discipline, overlapping with biology, chemistry, pharmacology, and medicine, that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnosing and treating exposures to toxins and toxicants. The relationship between dose and its effects on the exposed organism is of high significance in toxicology. Factors that influence chemical toxicity include the dosage, duration of exposure, route of exposure, species, age, sex, and environment. Toxicologists are experts on poisons and poisoning. There is a movement for evidence-based toxicology as part of the larger movement towards evidence-based practices. Toxicology is currently contributing to the field of cancer research, since some toxins can be used as drugs for killing tumor cells. One prime example of this is ribosome-inactivating proteins, tested in the treatment of leukemia.

A poison is any chemical substance that is harmful or lethal to living organisms. The term is used in a wide range of scientific fields and industries, where it is often specifically defined. It may also be applied colloquially or figuratively, with a broad sense.

Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a substructure of the organism, such as a cell (cytotoxicity) or an organ such as the liver (hepatotoxicity). Sometimes the word is more or less synonymous with poisoning in everyday usage.
The therapeutic index is a quantitative measurement of the relative safety of a drug with regard to risk of overdose. It is a comparison of the amount of a therapeutic agent that causes toxicity to the amount that causes the therapeutic effect. The related terms therapeutic window or safety window refer to a range of doses optimized between efficacy and toxicity, achieving the greatest therapeutic benefit without resulting in unacceptable side-effects or toxicity.

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.
In toxicology, the lethal dose (LD) is an indication of the lethal toxicity of a given substance or type of radiation. Because resistance varies from one individual to another, the "lethal dose" represents a dose at which a given percentage of subjects will die. The lethal concentration is a lethal dose measurement used for gases or particulates. The LD may be based on the standard person concept, a theoretical individual that has perfectly "normal" characteristics, and thus not apply to all sub-populations.
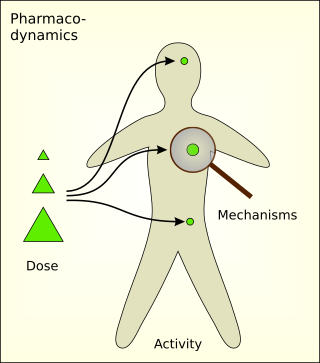
Pharmacodynamics (PD) is the study of the biochemical and physiologic effects of drugs. The effects can include those manifested within animals, microorganisms, or combinations of organisms.
Malathion is an organophosphate insecticide which acts as an acetylcholinesterase inhibitor. In the USSR, it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion.

Aquatic toxicology is the study of the effects of manufactured chemicals and other anthropogenic and natural materials and activities on aquatic organisms at various levels of organization, from subcellular through individual organisms to communities and ecosystems. Aquatic toxicology is a multidisciplinary field which integrates toxicology, aquatic ecology and aquatic chemistry.

Aldrin is an organochlorine insecticide that was widely used until the 1990s, when it was banned in most countries. Aldrin is a member of the so-called "classic organochlorines" (COC) group of pesticides. COCs enjoyed a very sharp rise in popularity during and after World War II. Other noteworthy examples of COCs include dieldrin and DDT. After research showed that organochlorines can be highly toxic to the ecosystem through bioaccumulation, most were banned from use. Before the ban, it was heavily used as a pesticide to treat seed and soil. Aldrin and related "cyclodiene" pesticides became notorious as persistent organic pollutants.
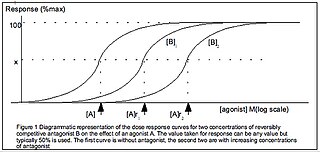
Half maximal effective concentration (EC50) is a measure of the concentration of a drug, antibody or toxicant which induces a biological response halfway between the baseline and maximum after a specified exposure time. More simply, EC50 can be defined as the concentration required to obtain a 50% [...] effect and may be also written as [A]50. It is commonly used as a measure of a drug's potency, although the use of EC50 is preferred over that of 'potency', which has been criticised for its vagueness. EC50 is a measure of concentration, expressed in molar units (M), where 1 M is equivalent to 1 mol/L.

The dose–response relationship, or exposure–response relationship, describes the magnitude of the response of an organism, as a function of exposure to a stimulus or stressor after a certain exposure time. Dose–response relationships can be described by dose–response curves. This is explained further in the following sections. A stimulus response function or stimulus response curve is defined more broadly as the response from any type of stimulus, not limited to chemicals.

Phosmet is a phthalimide-derived, non-systemic, organophosphate insecticide used on plants and animals. It is mainly used on apple trees for control of codling moth, though it is also used on a wide range of fruit crops, ornamentals, and vines for the control of aphids, suckers, mites, and fruit flies.
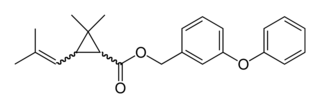
Phenothrin, also called sumithrin and d-phenothrin, is a synthetic pyrethroid that kills adult fleas and ticks. It has also been used to kill head lice in humans. d-Phenothrin is used as a component of aerosol insecticides for domestic use. It is often used with methoprene, an insect growth regulator that interrupts the insect's biological lifecycle by killing the eggs.
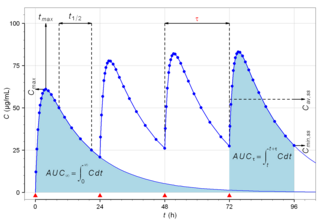
Pharmacokinetics, sometimes abbreviated as PK, is a branch of pharmacology dedicated to describing how the body affects a specific substance after administration. The substances of interest include any chemical xenobiotic such as pharmaceutical drugs, pesticides, food additives, cosmetics, etc. It attempts to analyze chemical metabolism and to discover the fate of a chemical from the moment that it is administered up to the point at which it is completely eliminated from the body. Pharmacokinetics is based on mathematical modeling that places great emphasis on the relationship between drug plasma concentration and the time elapsed since the drug's administration. Pharmacokinetics is the study of how an organism affects the drug, whereas pharmacodynamics (PD) is the study of how the drug affects the organism. Both together influence dosing, benefit, and adverse effects, as seen in PK/PD models.

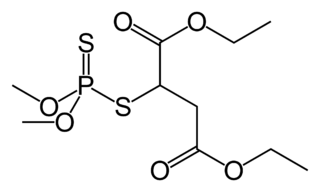
Sulfotep (also known as tetraethyldithiopyrophosphate and TEDP) is a pesticide commonly used in greenhouses as a fumigant. The substance is also known as Dithione, Dithiophos, and many other names. Sulfotep has the molecular formula C8H20O5P2S2 and belongs to the organophosphate class of chemicals. It has a cholinergic effect, involving depression of the cholinesterase activity of the peripheral and central nervous system of insects. The transduction of signals is disturbed at the synapses that make use of acetylcholine. Sulfotep is a mobile oil that is pale yellow-colored and smells like garlic. It is primarily used as an insecticide.
In aquatic toxicology, bioconcentration is the accumulation of a water-borne chemical substance in an organism exposed to the water.
Occupational toxicology is the application of toxicology to chemical hazards in the workplace. It focuses on substances and conditions that people may be exposed to in workplaces, including inhalation and dermal exposures, which are most prevalent when discussing occupational toxicology. These environmental and individual exposures can impact health, and there is a focus on identifying early adverse affects that are more subtle than those presented in clinical medicine.

Poisoning is the harmful effect which occurs when toxic substances are introduced into the body. The term "poisoning" is a derivative of poison, a term describing any chemical substance that may harm or kill a living organism upon ingestion. Poisoning can be brought on by swallowing, inhaling, injecting or absorbing toxins through the skin. Toxicology is the practice and study of symptoms, mechanisms, diagnoses, and treatments correlated to poisoning.


















