
In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are nonsuperposable onto their own mirror image. Enantiomers are much like one's right and left hands; without mirroring one of them, hands cannot be superposed onto each other. No amount of reorientation in three spatial dimensions will allow the four unique groups on the chiral carbon to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has.

In chemistry, a molecule or ion is called chiral if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality. The terms are derived from Ancient Greek χείρ (cheir) 'hand'; which is the canonical example of an object with this property.
Organic synthesis is a branch of chemical synthesis concerned with the construction of organic compounds. Organic compounds are molecules consisting of combinations of covalently-linked hydrogen, carbon, oxygen, and nitrogen atoms. Within the general subject of organic synthesis, there are many different types of synthetic routes that can be completed including total synthesis, stereoselective synthesis, automated synthesis, and many more. Additionally, in understanding organic synthesis it is necessary to be familiar with the methodology, techniques, and applications of the subject.

Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric products in unequal amounts."

In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ligare, which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure.

In the field of molecular modeling, docking is a method which predicts the preferred orientation of one molecule to a second when a ligand and a target are bound to each other to form a stable complex. Knowledge of the preferred orientation in turn may be used to predict the strength of association or binding affinity between two molecules using, for example, scoring functions.

Ronald Charles David Breslow was an American chemist from Rahway, New Jersey. He was University Professor at Columbia University, where he was based in the Department of Chemistry and affiliated with the Departments of Biological Sciences and Pharmacology; he had also been on the faculty of its Department of Chemical Engineering. He had taught at Columbia since 1956 and was a former chair of the university's chemistry department.
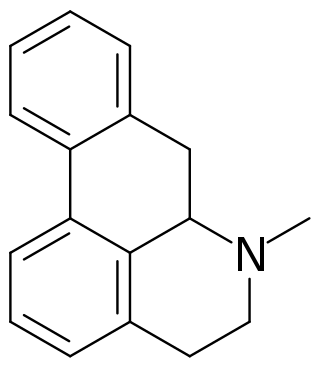
Aporphine is an alkaloid with the chemical formula C17H17N. It is the core chemical substructure of the aporphine alkaloids, a subclass of quinoline alkaloids. It can exist in either of two enantiomeric forms, (R)-aporphine and (S)-aporphine.

Epiboxidine is a chemical compound which acts as a partial agonist at neural nicotinic acetylcholine receptors, binding to both the α3β4 and the α4β2 subtypes. It was developed as a less toxic analogue of the potent frog-derived alkaloid epibatidine, which is around 200 times stronger than morphine as an analgesic but produces extremely dangerous toxic nicotinic side effects.

4-NEMD is a potent sedative drug which acts as a selective alpha-2 adrenergic agonist. It is closely related to dexmedetomidine but is several times more potent. Like other alpha-2 agonists, it produces sedative and muscle relaxant effects but without producing respiratory depression. It is not currently used in medicine but has been researched as the basis for a potential new generation of alpha-2 agonist drugs, which may have selectivity for the different subtypes of the alpha-2 receptor. It has two isomers, with the (S) isomer being the more potent, as with medetomidine. 4-NEMD was also investigated by the United States military as an anaesthetic agent, most likely for use in surgery but possibly also for use as a non-lethal incapacitating agent, although this has not been officially confirmed.
The eudysmic ratio represents the difference in pharmacologic activity between the two enantiomers of a drug. In most cases where a chiral compound is biologically active, one enantiomer is more active than the other. The eudysmic ratio is the ratio of activity between the two. A eudysmic ratio significantly differing from 1 means that they are statistically different in activity. Eudisimic ratio (ER) reflects the degree of enantioselectivity of the biological systems. For example, (S)-propranolol meaning that (S)-propranolol is 130 times more active than its (R)-enantiomer.

Melatonin receptor agonists are analogues of melatonin that bind to and activate the melatonin receptor. Agonists of the melatonin receptor have a number of therapeutic applications including treatment of sleep disorders and depression. The discovery and development of melatonin receptor agonists was motivated by the need for more potent analogues than melatonin, with better pharmacokinetics and longer half-lives. Melatonin receptor agonists were developed with the melatonin structure as a model.
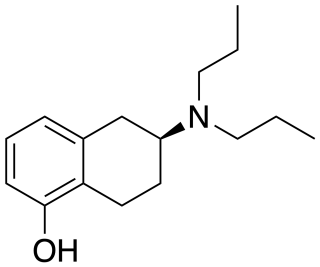
5-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with selectivity for the D2 receptor and D3 receptor subtypes. Only the (S)-enantiomer is active as an agonist, with the (R)-enantiomer being a weak antagonist at D2 receptors. Radiolabelled 11C-5-OH-DPAT is used as an agonist radioligand for mapping the distribution and function of D2 and D3 receptors in the brain, and the drug is also being studied in the treatment of Parkinson's disease.

Mitragynine pseudoindoxyl is a rearrangement product of 7-hydroxymitragynine and active metabolite of mitragynine. It is an analgesic being more potent than morphine.
In chemistry, a chalcogen bond (ChB) is an attractive interaction in the family of σ-hole interactions, along with halogen bonds. Electrostatic, charge-transfer (CT) and dispersion terms have been identified as contributing to this type of interaction. In terms of CT contribution, this family of attractive interactions has been modeled as an electron donor ) interacting with the σ* orbital of a C-X bond of the bond donor. In terms of electrostatic interactions, the molecular electrostatic potential (MEP) maps is often invoked to visualize the electron density of the donor and an electrophilic region on the acceptor, where the potential is depleted, referred to as a σ-hole. ChBs, much like hydrogen and halogen bonds, have been invoked in various non-covalent interactions, such as protein folding, crystal engineering, self-assembly, catalysis, transport, sensing, templation, and drug design.

Iwao Ojima is a Japanese-American chemist and university distinguished professor at the State University of New York at Stony Brook. He has been widely recognized for his seminal contributions to a range of chemical research at the multifaceted interfaces of chemical synthesis and life sciences. As rare accomplishments, he has received four National Awards from the American Chemical Society in four different fields of research. He is also serving as the director of the Institute of Chemical Biology and Drug Discovery (ICB&DD), as well as the president of the Stony Brook Chapter of the National Academy of Inventors.
A chiral switch is a chiral drug that has already approved as racemate but has been re-developed as a single enantiomer. The term chiral switching was introduced by Agranat and Caner in 1999 to describe the development of single enantiomers from racemate drugs. For example, levofloxacin is a chiral switch of racemic ofloxacin. The essential principle of a chiral switch is that there is a change in the status of chirality. In general, the term chiral switch is preferred over racemic switch because the switch is usually happening from a racemic drug to the corresponding single enantiomer(s). It is important to understand that chiral switches are treated as a selection invention. A selection invention is an invention that selects a group of new members from a previously known class on the basis of superior properties. To express the pharmacological activities of each of the chiral twins of a racemic drug two technical terms have been coined eutomer and distomer. The member of the chiral twin that has greater physiological activity is referred to as the eutomer and the other one with lesser activity is referred to as distomer. The eutomer/distomer ratio is called the eudisimic ratio and reflects the degree of enantioselectivity of the biological activity.
Chemical compounds that come as mirror-image pairs are referred to by chemists as chiral or handed molecules. Each twin is called an enantiomer. Drugs that exhibit handedness are referred to as chiral drugs. Chiral drugs that are equimolar (1:1) mixture of enantiomers are called racemic drugs and these are obviously devoid of optical rotation. The most commonly encountered stereogenic unit, that confers chirality to drug molecules are stereogenic center. Stereogenic center can be due to the presence of tetrahedral tetra coordinate atoms (C,N,P) and pyramidal tricoordinate atoms (N,S). The word chiral describes the three-dimensional architecture of the molecule and does not reveal the stereochemical composition. Hence "chiral drug" does not say whether the drug is racemic, single enantiomer or some other combination of stereoisomers. To resolve this issue Joseph Gal introduced a new term called unichiral. Unichiral indicates that the stereochemical composition of a chiral drug is homogenous consisting of a single enantiomer.
Chiral inversion is the process of conversion of one enantiomer of a chiral molecule to its mirror-image version with no other change in the molecule.
Chiral analysis refers to the quantification of component enantiomers of racemic drug substances or pharmaceutical compounds. Other synonyms commonly used include enantiomer analysis, enantiomeric analysis, and enantioselective analysis. Chiral analysis includes all analytical procedures focused on the characterization of the properties of chiral drugs. Chiral analysis is usually performed with chiral separation methods where the enantiomers are separated on an analytical scale and simultaneously assayed for each enantiomer.











