Related Research Articles
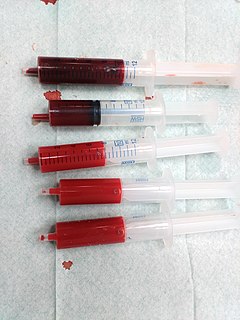
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells.

Hemoglobin, abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in red blood cells (erythrocytes) of almost all vertebrates as well as the tissues of some invertebrates. Hemoglobin in blood carries oxygen from the respiratory organs to the rest of the body. There it releases the oxygen to permit aerobic respiration to provide energy to power functions of an organism in the process called metabolism. A healthy individual human has 12 to 20 grams of hemoglobin in every 100 mL of blood.

Red blood cells (RBCs), also referred to as red cells, red blood corpuscles (in humans or other animals not having nucleus in red blood cells), haematids, erythroid cells or erythrocytes (from Greek erythros for "red" and kytos for "hollow vessel", with -cyte translated as "cell" in modern usage), are the most common type of blood cell and the vertebrate's principal means of delivering oxygen (O2) to the body tissues—via blood flow through the circulatory system. RBCs take up oxygen in the lungs, or in fish the gills, and release it into tissues while squeezing through the body's capillaries.

Hemoglobinopathy is the medical term for a group of inherited blood disorders and diseases that primarily affect red blood cells. They are single-gene disorders and, in most cases, they are inherited as autosomal co-dominant traits.

Methemoglobinemia, or methaemoglobinaemia, is a condition of elevated methemoglobin in the blood. Symptoms may include headache, dizziness, shortness of breath, nausea, poor muscle coordination, and blue-colored skin (cyanosis). Complications may include seizures and heart arrhythmias.

Cyanosis is the change of body tissue color to a bluish-purple hue as a result of having decreased amounts of oxygen bound to the hemoglobin in the red blood cells of the capillary bed. Body tissues that reflect cyanosis are usually in locations where the skin is thinner, including the mucous membranes, lips, nail beds, and ear lobes. Some medications containing amiodarone or silver, Mongolian spots, large birth marks, and the consumption of food products with blue or purple dyes can also result in the bluish skin tissue discoloration and may be mistaken for cyanosis.

Hereditary spherocytosis (HS) is a congenital hemolytic disorder, wherein a genetic mutation coding for a structural membrane protein phenotype leads to a spherical shaping of erythrocytic cellular morphology. As erythrocytes are sphere-shaped (spherocytosis), rather than the normal biconcave disk-shaped, their morphology interferes with these cells' abilities to be flexible during circulation throughout the entirety of the body - arteries, arterioles, capillaries, venules, veins, and organs. This difference in shape also makes the red blood cells more prone to rupture under osmotic and/or mechanical stress. Cells with these dysfunctional proteins are degraded in the spleen, which leads to a shortage of erythrocytes resulting in hemolytic anemia.
Fetal hemoglobin, or foetal haemoglobin is the main oxygen carrier protein in the human fetus. Hemoglobin F is found in fetal red blood cells, and is involved in transporting oxygen from the mother's bloodstream to organs and tissues in the fetus. It is produced at around 6 weeks of pregnancy and the levels remain high after birth until the baby is roughly 2–4 months old. Hemoglobin F has a different composition from the adult forms of hemoglobin, which allows it to bind oxygen more strongly. This way, the developing fetus is able to retrieve oxygen from the mother's bloodstream, which occurs through the placenta found in the mother's uterus.

Methemoglobin (British: methaemoglobin) (pronounced "met-hemoglobin") is a hemoglobin in the form of metalloprotein, in which the iron in the heme group is in the Fe3+ (ferric) state, not the Fe2+ (ferrous) of normal hemoglobin. Sometimes, it is also referred to as ferrihemoglobin. Methemoglobin cannot bind oxygen, which means it cannot carry oxygen to tissues. It is bluish chocolate-brown in color. In human blood a trace amount of methemoglobin is normally produced spontaneously, but when present in excess the blood becomes abnormally dark bluish brown. The NADH-dependent enzyme methemoglobin reductase (a type of diaphorase) is responsible for converting methemoglobin back to hemoglobin.
Glycated hemoglobin is a form of hemoglobin (Hb) that is chemically linked to a sugar. Most monosaccharides, including glucose, galactose and fructose, spontaneously bond with hemoglobin, when present in the bloodstream of humans. However, glucose is less likely to do so than galactose and fructose, which may explain why glucose is used as the primary metabolic fuel in humans.
Hemoglobin C is an abnormal hemoglobin in which glutamic acid residue at the 6th position of the β-globin chain is replaced with a lysine residue due to a point mutation in the HBB gene. People with one copy of the gene for hemoglobin C do not experience symptoms, but can pass the abnormal gene on to their children. Those with two copies of the gene are said to have hemoglobin C disease and can experience mild anemia. It is possible for a person to have both the gene for hemoglobin S and the gene for hemoglobin C; this state is called hemoglobin SC disease, and is generally more severe than hemoglobin C disease, but milder than sickle cell anemia.

A CO-oximeter is a device that measures the oxygen carrying state of hemoglobin in a blood specimen, including oxygen-carrying hemoglobin (O2Hb), non-oxygen-carrying but normal hemoglobin (HHb), as well as the dyshemoglobins such as carboxyhemoglobin (COHb) and methemoglobin (MetHb). The use of 'CO' rather than 'Co' or 'co' is more appropriate since this designation represents a device that measures carbon monoxide (CO) bound to hemoglobin, as distinguished from simple oximetry which measures hemoglobin bound to molecular oxygen—O2Hb—or hemoglobin capable of binding to molecular oxygen—HHb. Simpler oximeters may report oxygen saturation alone, i.e. the ratio of oxyhemoglobin to total 'bindable' hemoglobin. CO-oximetry is useful in defining the causes for hypoxemia, or hypoxia,.
The oxygen–hemoglobin dissociation curve, also called the oxyhemoglobin dissociation curve or oxygen dissociation curve (ODC), is a curve that plots the proportion of hemoglobin in its saturated (oxygen-laden) form on the vertical axis against the prevailing oxygen tension on the horizontal axis. This curve is an important tool for understanding how our blood carries and releases oxygen. Specifically, the oxyhemoglobin dissociation curve relates oxygen saturation (SO2) and partial pressure of oxygen in the blood (PO2), and is determined by what is called "hemoglobin affinity for oxygen"; that is, how readily hemoglobin acquires and releases oxygen molecules into the fluid that surrounds it.
The Haldane effect is a property of hemoglobin first described by John Scott Haldane, within which oxygenation of blood in the lungs displaces carbon dioxide from hemoglobin, increasing the removal of carbon dioxide. Consequently, oxygenated blood has a reduced affinity for carbon dioxide. Thus, the Haldane effect describes the ability of hemoglobin to carry increased amounts of carbon dioxide (CO2) in the deoxygenated state as opposed to the oxygenated state. A high concentration of CO2 facilitates dissociation of oxyhemoglobin.
Heinz bodies are inclusions within red blood cells composed of denatured hemoglobin. They are not visible with routine blood staining techniques, but can be seen with supravital staining. The presence of Heinz bodies represents damage to hemoglobin and is classically observed in G6PD deficiency, a genetic disorder that causes hemolytic anemia. In veterinary medicine, Heinz bodies may be seen following the consumption of foods containing thiosulfate and propylene glycol compounds by cats, dogs and certain primates.

2,3-Bisphosphoglyceric acid (2,3-BPG), also known as 2,3-diphosphoglyceric acid (2,3-DPG), is a three-carbon isomer of the glycolytic intermediate 1,3-bisphosphoglyceric acid (1,3-BPG).

Rouleaux are stacks or aggregations of red blood cells (RBCs) that form because of the unique discoid shape of the cells in vertebrates. The flat surface of the discoid RBCs gives them a large surface area to make contact with and stick to each other; thus forming a rouleau. They occur when the plasma protein concentration is high, and, because of them, the ESR is also increased. This is a nonspecific indicator of the presence of disease.
Sulfhemoglobinemia is a rare condition in which there is excess sulfhemoglobin (SulfHb) in the blood. The pigment is a greenish derivative of hemoglobin which cannot be converted back to normal, functional hemoglobin. It causes cyanosis even at low blood levels.
Erythrocyte deformability refers to the ability of erythrocytes to change shape under a given level of applied stress, without hemolysing (rupturing). This is an important property because erythrocytes must change their shape extensively under the influence of mechanical forces in fluid flow or while passing through microcirculation. The extent and geometry of this shape change can be affected by the mechanical properties of the erythrocytes, the magnitude of the applied forces, and the orientation of erythrocytes with the applied forces. Deformability is an intrinsic cellular property of erythrocytes determined by geometric and material properties of the cell membrane, although as with many measurable properties the ambient conditions may also be relevant factors in any given measurement. No other cells of mammalian organisms have deformability comparable with erythrocytes; furthermore, non-mammalian erythrocytes are not deformable to an extent comparable with mammalian erythrocytes. In human RBC there are structural support that aids resilience in RBC which include the cytoskeleton- actin and spectrin that are held together by ankyrin.

Erythrocyte aggregation is the reversible clumping of red blood cells (RBCs) under low shear forces or at stasis.
References
- ↑ Riccio A.; Vitagliano L.; di Prisco G.; Zagari A.; Mazzarella L. (2002). "The crystal structure of a tetrameric hemoglobin in a partial hemichrome state". Proceedings of the National Academy of Sciences of the United States of America . 99 (15): 9801–9806. Bibcode:2002PNAS...99.9801R. doi: 10.1073/pnas.132182099 . PMC 125021 . PMID 12093902.
- 1 2 3 4 Molchanova, T. http://www.tatianamolchanova.com/files/Hemichrome_alphaHb_AHSP_Molchanova.pdf
- ↑ Kannan, R., Labotka, R., & Low, P.S. (1988). "Isolation and characterization of the hemichrome-stabilized membrane protein aggregates from sickle erythrocytes". The Journal of Biological Chemistry . 263 (27): 13766–13773. doi: 10.1016/S0021-9258(18)68308-5 .
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Sugawara, Y. Y., Kadono, E. E., Suzuki, A. A., Yukuta, Y. Y., Shibasaki, Y. Y., Nishimura, N. N.; et al. (2003). "Hemichrome formation observed in human hemoglobin A under various buffer conditions". Acta Physiologica Scandinavica . 179 (1): 49–59. doi:10.1046/j.1365-201X.2003.01142.x. PMID 12940938.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Mannu, F., Arese, P., Cappellini, M.D., Fiorelli, G., Cappadoro, M., Giribaldi, G.; et al. (1995). "Role of hemichrome binding to erythrocyte membrane in the generation of band-3 alterations in β-thalassemia intermedia erythrocytes". Blood . 86 (5): 2014–2020. doi: 10.1182/blood.V86.5.2014.bloodjournal8652014 . PMID 7655029.
{{cite journal}}: CS1 maint: multiple names: authors list (link)