
Gadolinium is a chemical element with the symbol Gd and atomic number 64. Gadolinium is a silvery-white metal when oxidation is removed. It is only slightly malleable and is a ductile rare-earth element. Gadolinium reacts with atmospheric oxygen or moisture slowly to form a black coating. Gadolinium below its Curie point of 20 °C (68 °F) is ferromagnetic, with an attraction to a magnetic field higher than that of nickel. Above this temperature it is the most paramagnetic element. It is found in nature only in an oxidized form. When separated, it usually has impurities of the other rare-earths because of their similar chemical properties.

Ferrofluid is a liquid that is attracted to the poles of a magnet. It is a colloidal liquid made of nanoscale ferromagnetic or ferrimagnetic particles suspended in a carrier fluid. Each magnetic particle is thoroughly coated with a surfactant to inhibit clumping. Large ferromagnetic particles can be ripped out of the homogeneous colloidal mixture, forming a separate clump of magnetic dust when exposed to strong magnetic fields. The magnetic attraction of tiny nanoparticles is weak enough that the surfactant's Van der Waals force is sufficient to prevent magnetic clumping or agglomeration. Ferrofluids usually do not retain magnetization in the absence of an externally applied field and thus are often classified as "superparamagnets" rather than ferromagnets.
Dynamic nuclear polarization (DNP) results from transferring spin polarization from electrons to nuclei, thereby aligning the nuclear spins to the extent that electron spins are aligned. Note that the alignment of electron spins at a given magnetic field and temperature is described by the Boltzmann distribution under the thermal equilibrium. It is also possible that those electrons are aligned to a higher degree of order by other preparations of electron spin order such as: chemical reactions, optical pumping and spin injection. DNP is considered one of several techniques for hyperpolarization. DNP can also be induced using unpaired electrons produced by radiation damage in solids.
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.

Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford.
Montonen–Olive duality or electric–magnetic duality is the oldest known example of strong–weak duality or S-duality according to current terminology. It generalizes the electro-magnetic symmetry of Maxwell's equations by stating that magnetic monopoles, which are usually viewed as emergent quasiparticles that are "composite", can in fact be viewed as "elementary" quantized particles with electrons playing the reverse role of "composite" topological solitons; the viewpoints are equivalent and the situation dependent on the duality. It was later proven to hold true when dealing with a N = 4 supersymmetric Yang–Mills theory. It is named after Finnish physicist Claus Montonen and British physicist David Olive after they proposed the idea in their academic paper Magnetic monopoles as gauge particles? where they state:
There should be two "dual equivalent" field formulations of the same theory in which electric (Noether) and magnetic (topological) quantum numbers exchange roles.
Ferromagnetic resonance, or FMR, is coupling between an electromagnetic wave and the magnetization of a medium through which it passes. This coupling induces a significant loss of power of the wave. The power is absorbed by the precessing magnetization of the material and lost as heat. For this coupling to occur, the frequency of the incident wave must be equal to the precession frequency of the magnetization and the polarization of the wave must match the orientation of the magnetization.

Magnetic resonance microscopy is magnetic resonance imaging (MRI) at a microscopic level down to the scale of microns. The first definition of MRM was MRI having voxel resolutions of better than 100 μm.
Jozef T. Devreese is a Belgian scientist, with a long career in condensed matter physics. He is Professor Emeritus of Theoretical Physics at the University of Antwerp.

Helimagnetism is a form of magnetic ordering where spins of neighbouring magnetic moments arrange themselves in a spiral or helical pattern, with a characteristic turn angle of somewhere between 0 and 180 degrees. It results from the competition between ferromagnetic and antiferromagnetic exchange interactions. It is possible to view ferromagnetism and antiferromagnetism as helimagnetic structures with characteristic turn angles of 0 and 180 degrees respectively. Helimagnetic order breaks spatial inversion symmetry, as it can be either left-handed or right-handed in nature.

The nitrogen-vacancy center is one of numerous point defects in diamond. Its most explored and useful property is its photoluminescence, which allows observers to read out its spin-state. The NV center's electron spin, localized at atomic scales, can be manipulated at room temperature by external factors such as magnetic, or electric fields, microwave radiation, or optical light, resulting in sharp resonances in the intensity of the photoluminescence. These resonances can be explained in terms of electron spin related phenomena such as quantum entanglement, spin–orbit interaction and Rabi oscillations, and analysed using advanced quantum optics theory. An individual NV center can be used as a basic unit for a quantum computer, a qubit, and used for quantum cryptography. Further potential applications in novel fields of electronics and sensing include spintronics, masers, and quantum sensors. If the charge is not specified the term "NV center" refers to the negatively charged NV− center.
The term magnetic structure of a material pertains to the ordered arrangement of magnetic spins, typically within an ordered crystallographic lattice. Its study is a branch of solid-state physics.
A Mott transition is a metal-nonmetal transition in condensed matter. Due to electric field screening the potential energy becomes much more sharply (exponentially) peaked around the equilibrium position of the atom and electrons become localized and can no longer conduct a current.
Electron nuclear double resonance (ENDOR) is a magnetic resonance technique for elucidating the molecular and electronic structure of paramagnetic species. The technique was first introduced to resolve interactions in electron paramagnetic resonance (EPR) spectra. It is currently practiced in a variety of modalities, mainly in the areas of biophysics and heterogeneous catalysis.
Acoustic paramagnetic resonance (APR) is a phenomenon of resonant absorption of sound by a system of magnetic particles placed in an external magnetic field. It occurs when the energy of the sound wave quantum becomes equal to the splitting of the energy levels of the particles, the splitting being induced by the magnetic field. APR is a variation of electron paramagnetic resonance (EPR) where the acoustic rather than electromagnetic waves are absorbed by the studied sample. APR was theoretically predicted in 1952, independently by Semen Altshuler and Alfred Kastler, and was experimentally observed by W. G. Proctor and W. H. Tanttila in 1955.
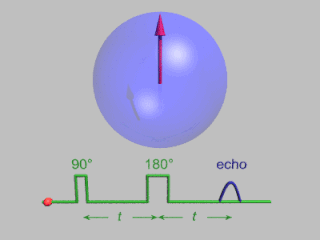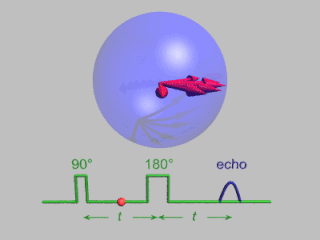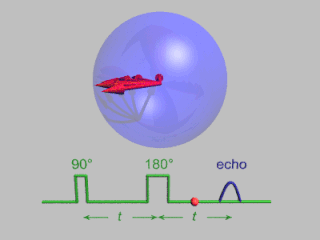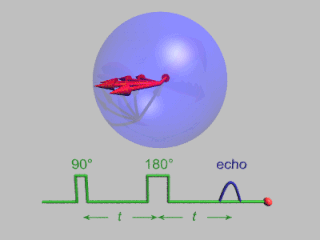
Pulsed electron paramagnetic resonance (EPR) is an electron paramagnetic resonance technique that involves the alignment of the net magnetization vector of the electron spins in a constant magnetic field. This alignment is perturbed by applying a short oscillating field, usually a microwave pulse. One can then measure the emitted microwave signal which is created by the sample magnetization. Fourier transformation of the microwave signal yields an EPR spectrum in the frequency domain. With a vast variety of pulse sequences it is possible to gain extensive knowledge on structural and dynamical properties of paramagnetic compounds. Pulsed EPR techniques such as electron spin echo envelope modulation (ESEEM) or pulsed electron nuclear double resonance (ENDOR) can reveal the interactions of the electron spin with its surrounding nuclear spins.
The Gas Dynamic Trap is a magnetic mirror machine being operated at the Budker Institute of Nuclear Physics in Akademgorodok, Russia.

Wolfgang Lubitz is a German chemist and biophysicist. He is currently a director emeritus at the Max Planck Institute for Chemical Energy Conversion. He is well known for his work on bacterial photosynthetic reaction centres, hydrogenase enzymes, and the oxygen-evolving complex using a variety of biophysical techniques. He has been recognized by a Festschrift for his contributions to electron paramagnetic resonance (EPR) and its applications to chemical and biological systems.
Hartmut Löwen is a German physicist working in the field of statistical mechanics and soft matter physics.
Caesium sesquioxide is a chemical compound with the formula Cs2O3 or Cs4O6. In terms of oxidation states, Caesium in this compound has a nominal charge of +1, and the oxygen is a mixed peroxide and superoxide for a structural formula of (Cs+)4(O−2)2(O2−2). Compared to the other caesium oxides, this phase is less well studied, but has been long present in the literature. It can be created by thermal decomposition of caesium superoxide at 290 °C.








