
Fibronectin is a high-molecular weight glycoprotein of the extracellular matrix that binds to membrane-spanning receptor proteins called integrins. Fibronectin also binds to other extracellular matrix proteins such as collagen, fibrin, and heparan sulfate proteoglycans.

In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.
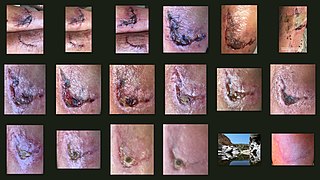
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.
A chronic wound is a wound that does not heal in an orderly set of stages and in a predictable amount of time the way most wounds do; wounds that do not heal within three months are often considered chronic. Chronic wounds seem to be detained in one or more of the phases of wound healing. For example, chronic wounds often remain in the inflammatory stage for too long. To overcome that stage and jump-start the healing process, a number of factors need to be addressed such as bacterial burden, necrotic tissue, and moisture balance of the whole wound. In acute wounds, there is a precise balance between production and degradation of molecules such as collagen; in chronic wounds this balance is lost and degradation plays too large a role.
Fibroblast growth factors (FGF) are a family of cell signalling proteins produced by macrophages; they are involved in a wide variety of processes, most notably as crucial elements for normal development in animal cells. Any irregularities in their function lead to a range of developmental defects. These growth factors typically act as systemic or locally circulating molecules of extracellular origin that activate cell surface receptors. A defining property of FGFs is that they bind to heparin and to heparan sulfate. Thus, some are sequestered in the extracellular matrix of tissues that contains heparan sulfate proteoglycans and are released locally upon injury or tissue remodeling.
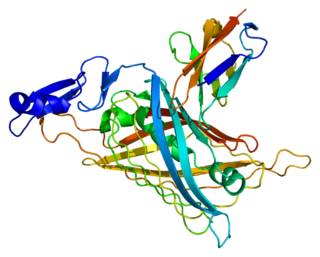
Perlecan (PLC) also known as basement membrane-specific heparan sulfate proteoglycan core protein (HSPG) or heparan sulfate proteoglycan 2 (HSPG2), is a protein that in humans is encoded by the HSPG2 gene. The HSPG2 gene codes for a 4,391 amino acid protein with a molecular weight of 468,829. It is one of the largest known proteins. The name perlecan comes from its appearance as a "string of pearls" in rotary shadowed images.

Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs as a proteoglycan in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB, and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 Infection, particularly when the virus attaches with ACE2.

Betaglycan also known as Transforming growth factor beta receptor III (TGFBR3), is a cell-surface chondroitin sulfate / heparan sulfate proteoglycan >300 kDa in molecular weight. Betaglycan binds to various members of the TGF-beta superfamily of ligands via its core protein, and bFGF via its heparan sulfate chains. TGFBR3 is the most widely expressed type of TGF-beta receptor. Its affinity towards all individual isoforms of TGF-beta is similarly high and therefore it plays an important role as a coreceptor mediating the binding of TGF-beta to its other receptors - specifically TGFBR2. The intrinsic kinase activity of this receptor has not yet been described. In regard of TGF-beta signalling it is generally considered a non-signaling receptor or a coreceptor. By binding to various member of the TGF-beta superfamily at the cell surface it acts as a reservoir of TGF-beta.

Syndecan 1 is a protein which in humans is encoded by the SDC1 gene. The protein is a transmembrane heparan sulfate proteoglycan and is a member of the syndecan proteoglycan family. The syndecan-1 protein functions as an integral membrane protein and participates in cell proliferation, cell migration and cell-matrix interactions via its receptor for extracellular matrix proteins. Syndecan-1 is a sponge for growth factors and chemokines, with binding largely via heparan sulfate chains. The syndecans mediate cell binding, cell signaling, and cytoskeletal organization and syndecan receptors are required for internalization of the HIV-1 tat protein.

Syndecans are single transmembrane domain proteins that are thought to act as coreceptors, especially for G protein-coupled receptors. More specifically, these core proteins carry three to five heparan sulfate and chondroitin sulfate chains, i.e. they are proteoglycans, which allow for interaction with a large variety of ligands including fibroblast growth factors, vascular endothelial growth factor, transforming growth factor-beta, fibronectin and antithrombin-1. Interactions between fibronectin and some syndecans can be modulated by the extracellular matrix protein tenascin C.

Matrilysin also known as matrix metalloproteinase-7 (MMP-7), pump-1 protease (PUMP-1), or uterine metalloproteinase is an enzyme in humans that is encoded by the MMP7 gene. The enzyme has also been known as matrin, putative metalloproteinase-1, matrix metalloproteinase pump 1, PUMP-1 proteinase, PUMP, metalloproteinase pump-1, putative metalloproteinase, MMP). Human MMP-7 has a molecular weight around 30 kDa.

Cysteine-rich angiogenic inducer 61 (CYR61) or CCN family member 1 (CCN1), is a matricellular protein that in humans is encoded by the CYR61 gene.

Heparanase, also known as HPSE, is an enzyme that acts both at the cell-surface and within the extracellular matrix to degrade polymeric heparan sulfate molecules into shorter chain length oligosaccharides.

Sulfatase 1, also known as SULF1, is an enzyme which in humans is encoded by the SULF1 gene.
A fibrin scaffold is a network of protein that holds together and supports a variety of living tissues. It is produced naturally by the body after injury, but also can be engineered as a tissue substitute to speed healing. The scaffold consists of naturally occurring biomaterials composed of a cross-linked fibrin network and has a broad use in biomedical applications.
Acellular dermis is a type of biomaterial derived from processing human or animal tissues to remove cells and retain portions of the extracellular matrix (ECM). These materials are typically cell-free, distinguishing them from classical allografts and xenografts, can be integrated or incorporated into the body, and have been FDA approved for human use for more than 10 years in a wide range of clinical indications.

In biochemistry, carbohydrate sulfotransferases are enzymes within the class of sulfotransferases which catalyze the transfer of the sulfate functional group to carbohydrate groups in glycoproteins and glycolipids. Carbohydrates are used by cells for a wide range of functions from structural purposes to extracellular communication. Carbohydrates are suitable for such a wide variety of functions due to the diversity in structure generated from monosaccharide composition, glycosidic linkage positions, chain branching, and covalent modification. Possible covalent modifications include acetylation, methylation, phosphorylation, and sulfation. Sulfation, performed by carbohydrate sulfotransferases, generates carbohydrate sulfate esters. These sulfate esters are only located extracellularly, whether through excretion into the extracellular matrix (ECM) or by presentation on the cell surface. As extracellular compounds, sulfated carbohydrates are mediators of intercellular communication, cellular adhesion, and ECM maintenance.

Decellularization is the process used in biomedical engineering to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells, leaving an ECM scaffold of the original tissue, which can be used in artificial organ and tissue regeneration. Organ and tissue transplantation treat a variety of medical problems, ranging from end organ failure to cosmetic surgery. One of the greatest limitations to organ transplantation derives from organ rejection caused by antibodies of the transplant recipient reacting to donor antigens on cell surfaces within the donor organ. Because of unfavorable immune responses, transplant patients suffer a lifetime taking immunosuppressing medication. Stephen F. Badylak pioneered the process of decellularization at the McGowan Institute for Regenerative Medicine at the University of Pittsburgh. This process creates a natural biomaterial to act as a scaffold for cell growth, differentiation and tissue development. By recellularizing an ECM scaffold with a patient’s own cells, the adverse immune response is eliminated. Nowadays, commercially available ECM scaffolds are available for a wide variety of tissue engineering. Using peracetic acid to decellularize ECM scaffolds have been found to be false and only disinfects the tissue.

Diabetic foot ulcer is a breakdown of the skin and sometimes deeper tissues of the foot that leads to sore formation. It may occur due to a variety of mechanisms. It is thought to occur due to abnormal pressure or mechanical stress chronically applied to the foot, usually with concomitant predisposing conditions such as peripheral sensory neuropathy, peripheral motor neuropathy, autonomic neuropathy or peripheral arterial disease. It is a major complication of diabetes mellitus, and it is a type of diabetic foot disease. Secondary complications to the ulcer, such as infection of the skin or subcutaneous tissue, bone infection, gangrene or sepsis are possible, often leading to amputation.

Ovine forestomach matrix (OFM) is a layer of decellularized extracellular matrix (ECM) biomaterial isolated from the propria submucosa of the rumen of sheep. OFM is used in tissue engineering and as a tissue scaffold for wound healing and surgical applications

















