| Look up heterogeneity or heterogeneous in Wiktionary, the free dictionary. |
Heterogeneity is a diverseness of constituent structure.
Contents
Heterogeneity or heterogeneous may also refer to:
| Look up heterogeneity or heterogeneous in Wiktionary, the free dictionary. |
Heterogeneity is a diverseness of constituent structure.
Heterogeneity or heterogeneous may also refer to:
| This disambiguation page lists articles associated with the title Heterogeneity. If an internal link led you here, you may wish to change the link to point directly to the intended article. |

A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei, and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur.
Chromatography is a laboratory technique for the separation of a mixture. The mixture is dissolved in a fluid called the mobile phase, which carries it through a system on which is fixed a material called the stationary phase. The different constituents of the mixture have different affinities for the stationary phase. The different molecules stay longer or shorter on the stationary phase, depending on their interactions with its surface sites. So, they travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation.

In the physical sciences, a phase is a region of space, throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase.

In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when one of the solvents is water.

In physics, a state of matter is one of the distinct forms in which matter can exist. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many intermediate states are known to exist, such as liquid crystal, and some states only exist under extreme conditions, such as Bose–Einstein condensates, neutron-degenerate matter, and quark–gluon plasma, which only occur, respectively, in situations of extreme cold, extreme density, and extremely high energy. For a complete list of all exotic states of matter, see the list of states of matter.

In chemistry, thermodynamics, and many other related fields, phase transitions are the physical processes of transition between the basic states of matter: solid, liquid, and gas, as well as plasma in rare cases.
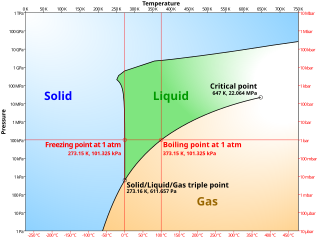
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions at which thermodynamically distinct phases occur and coexist at equilibrium.
Surface science is the study of physical and chemical phenomena that occur at the interface of two phases, including solid–liquid interfaces, solid–gas interfaces, solid–vacuum interfaces, and liquid–gas interfaces. It includes the fields of surface chemistry and surface physics. Some related practical applications are classed as surface engineering. The science encompasses concepts such as heterogeneous catalysis, semiconductor device fabrication, fuel cells, self-assembled monolayers, and adhesives. Surface science is closely related to interface and colloid science. Interfacial chemistry and physics are common subjects for both. The methods are different. In addition, interface and colloid science studies macroscopic phenomena that occur in heterogeneous systems due to peculiarities of interfaces.
Homogeneity is a sameness of constituent structure.
In chemistry, a mixture is a material made up of two or more different substances which are physically combined. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids.

In industrial process engineering, mixing is a unit operation that involves manipulation of a heterogeneous physical system with the intent to make it more homogeneous. Familiar examples include pumping of the water in a swimming pool to homogenize the water temperature, and the stirring of pancake batter to eliminate lumps (deagglomeration).
A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.
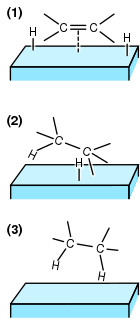
In chemistry, heterogeneous catalysis is catalysis where the phase of catalysts differs from that of the reactants or products. The process contrasts with homogeneous catalysis where the reactants, products and catalyst exist in the same phase. Phase distinguishes between not only solid, liquid, and gas components, but also immiscible mixtures, or anywhere an interface is present. Catalysts are useful because they increase the rate of a reaction without themselves being consumed and are therefore reusable.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides.
Homogeneity and heterogeneity are concepts often used in the sciences and statistics relating to the uniformity of a substance or organism. A material or image that is homogeneous is uniform in composition or character ; one that is heterogeneous is distinctly nonuniform in one of these qualities.
The Freundlich equation or Freundlich adsorption isotherm, an adsorption isotherm, is an empirical relationship between the quantity of a gas adsorbed into a solid surface and the gas pressure. The same relationship is also applicable for the concentration of a solute adsorbed onto the surface of a solid and the concentration of the solute in the liquid phase. In 1909, Herbert Freundlich gave an expression representing the isothermal variation of adsorption of a quantity of gas adsorbed by unit mass of solid adsorbent with gas pressure. This equation is known as Freundlich adsorption isotherm or Freundlich adsorption equation. As this relationship is entirely empirical, in the case where adsorption behavior can be properly fit by isotherms with a theoretical basis, it is usually appropriate to use such isotherms instead. The Freundlich equation is also derived (non-empirically) by attributing the change in the equilibrium constant of the binding process to the heterogeneity of the surface and the variation in the heat of adsorption.
This glossary of chemistry terms is a list of terms and definitions relevant to chemistry, including chemical laws, diagrams and formulae, laboratory tools, glassware, and equipment. Chemistry is a physical science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions; it features an extensive vocabulary and a significant amount of jargon.
Industrial agitators are machines used in industries that process products in the chemical, food, pharmaceutical and cosmetic industries, in a view of :
The strength of metal oxide adhesion effectively determines the wetting of the metal-oxide interface. The strength of this adhesion is important, for instance, in production of light bulbs and fiber-matrix composites that depend on the optimization of wetting to create metal-ceramic interfaces. The strength of adhesion also determines the extent of dispersion on catalytically active metal. Metal oxide adhesion is important for applications such as complementary metal oxide semiconductor devices. These devices make possible the high packing densities of modern integrated circuits.