
Imidazole (ImH) is an organic compound with the formula (CH)3(NH)N. It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole.

Benzimidazole is a heterocyclic aromatic organic compound. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and imidazole. It is a white solid that appears in form of tabular crystals.
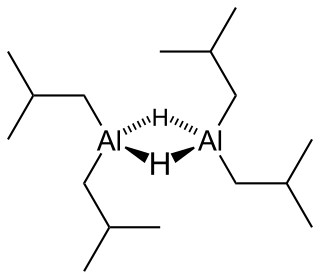
Diisobutylaluminium hydride (DIBALH, DIBAL, DIBAL-H or DIBAH) is a reducing agent with the formula (i-Bu2AlH)2, where i-Bu represents isobutyl (-CH2CH(CH3)2). This organoaluminium compound is a reagent in organic synthesis.

Photochromism is the reversible change of color upon exposure to light. It is a transformation of a chemical species (photoswitch) between two forms by the absorption of electromagnetic radiation (photoisomerization), where the two forms have different absorption spectra.

Hydrogen telluride is the inorganic compound with the formula H2Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.

Platinum(II) chloride describes the inorganic compounds with the formula PtCl2. They are precursor used in the preparation of other platinum compounds. Platinum(II) chloride exists in two crystalline forms (polymorphs), but the main properties are somewhat similar: dark brown, insoluble in water, diamagnetic, and odorless.

Imidazolidine is a heterocyclic compound (CH2)2(NH)2CH2. The parent imidazolidine is lightly studied, but related compounds substituted on one or both nitrogen centers are more common. Generally, they are colorless, polar, basic compounds. Imidazolidines are cyclic 5-membered examples of the general class of aminals.

Triacetonamine is an organic compound with the formula OC(CH2CMe2)2NH (where Me = CH3). It is a colorless or white solid that melts near room temperature. The compound is an intermediate in the preparation of 2,2,6,6-tetramethylpiperidine, a sterically hindered base and precursor to the reagent called TEMPO. Triacetonamine is formed by the poly-aldol condensation of acetone in the presence of ammonia and calcium chloride:

Imidazol-4-one-5-propionic acid is an intermediate in the metabolism of histidine. It is a colorless compound that is sensitive to light in air. The compound features an imidazolone ring.

Indane or indan is an organic compound with the formula C9H10. It is a colorless liquid hydrocarbon. It is a petrochemical, a bicyclic compound. It occurs at the level of about 0.1% in coal tar. It is usually produced by hydrogenation of indene.
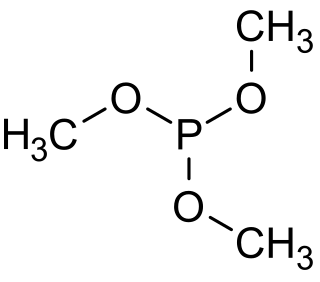
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is the simplest phosphite ester and finds used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.

Dehydroacetic acid is an organic compound which has several industrial applications. The compound is classified as a pyrone derivative. It presents as an odorless, colorless to white crystalline powder, almost insoluble in water and moderately soluble in most organic solvents.

1-Methylimidazole or N-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine.

1,3-Indandione (sometimes simply indanedione) is an organic compound with the molecular formula C6H4(CO)2CH2. It is a β-diketone with indane as its structural nucleus. It is a colorless or white solid, but old samples can appear yellowish or even green. It is a popular chemical scaffold (building block of various useful chemical compounds).

Imidazole-1-sulfonyl azide is an organic azide compound that can be used as an alternative organic synthesis reagent to trifluoromethanesulfonyl azide. It is an explosive colorless liquid, but some of its organic-soluble salts can be safely handled and stored as a solid.
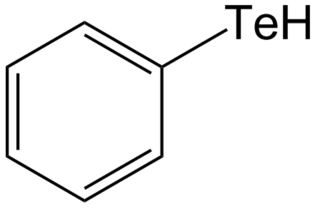
Tellurols are analogues of alcohols and phenols where tellurium replaces oxygen. Tellurols, selenols, and thiols have similar properties, but tellurols are the least stable. Although they are fundamental representatives of organotellurium compounds, tellurols are lightly studied because of their instability. Tellurol derivatives include telluroesters and tellurocyanates (RTeCN).

3-Chloropropionitrile is an organic compound with the formula ClCH2CH2CN. A colorless liquid, it is prepared by the reaction of hydrogen chloride with acrylonitrile. It is used commercially as a precursor to the drug famotidine.
A spiropyran is a type of organic chemical compound, known for photochromic properties that provide this molecule with the ability of being used in medical and technological areas. Spiropyrans were discovered in the early twentieth century. However, it was in the middle twenties when Fisher and Hirshbergin observed their photochromic characteristics and reversible reaction. In 1952, Fisher and co-workers announced for the first time photochromism in spiropyrans. Since then, there have been many studies on photochromic compounds that have continued up to the present.

2-Methylimidazole is an organic compound that is structurally related to imidazole with the chemical formula CH3C3H2N2H. It is a white or colorless solid that is highly soluble in polar organic solvents and water. It is a precursor to a range of drugs and is a ligand in coordination chemistry.

In organic chemistry, a fulgide is any of a class of photochromic compounds consisting of a bismethylene-succinic anhydride core that has an aromatic group as a substituent. The highly conjugated system is a good chromophore. It can undergo reversible photoisomerization induced by ultraviolet light, converting between the E and Z isomers, both of which are typically colorless compounds. Unlike the more-stable Z isomer, the E isomer can also undergo a photochemically-induced electrocyclic reaction, forming a new ring and becoming a distinctly colored product called the C form. It is thus the two-step Z–C isomerization that is the photochromic change starting from the stable uncyclized form.



















