
Carotenoids are yellow, orange, and red organic pigments that are produced by plants and algae, as well as several bacteria, archaea, and fungi. Carotenoids give the characteristic color to pumpkins, carrots, parsnips, corn, tomatoes, canaries, flamingos, salmon, lobster, shrimp, and daffodils. Over 1,100 identified carotenoids can be further categorized into two classes – xanthophylls and carotenes.

Scilloideae is a subfamily of bulbous plants within the family Asparagaceae. Scilloideae is sometimes treated as a separate family Hyacinthaceae, named after the genus Hyacinthus. Scilloideae or Hyacinthaceae include many familiar garden plants such as Hyacinthus (hyacinths), Hyacinthoides (bluebells), Muscari and Scilla and Puschkinia. Some are important as cut flowers.

Persicaria odorata, with common names Vietnamese coriander, rau răm, laksa leaf, Vietnamese cilantro, phak phai, praew leaf, hot mint, Cambodian mint and Vietnamese mint, is an herb whose leaves are used in Southeast Asian and Northeast Indian cooking.
The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."

Phenanthrenoids are chemical compounds formed with a phenanthrene backbone. These compounds occur naturally in plants, although they can also be synthesized.

Eucomis autumnalis, the autumn pineapple flower, or autumn pineapple lily, is a species of flowering plant in the family Asparagaceae, subfamily Scilloideae, native to Malawi, Zimbabwe and southern Africa. It is a mid to late summer flowering deciduous bulbous perennial. The flower stem reaches about 40 cm (16 in), rising from a basal rosette of wavy-edged leaves. The green, yellow or white flowers are arranged in a spike (raceme), topped by a "head" of green leaflike bracts. It is grown as an ornamental garden plant and can also be used as a cut flower.
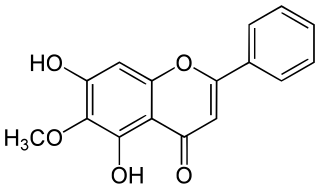
Oroxylin A is an O-methylated flavone, a chemical compound that can be found in the medicinal plants Scutellaria baicalensis and Scutellaria lateriflora, and the Oroxylum indicum tree. It has demonstrated activity as a dopamine reuptake inhibitor, and is also a negative allosteric modulator of the benzodiazepine site of the GABAA receptor. Oroxylin A has been found to improve memory consolidation in mice by elevating brain-derived neurotrophic factor (BDNF) levels in the hippocampus.

Aegiceras corniculatum, commonly known as black mangrove, river mangrove, goat's horn mangrove, or khalsi, is a species of shrub or tree mangrove in the primrose family, Primulaceae, with a distribution in coastal and estuarine areas ranging from India through South East Asia to southern China, New Guinea and Australia.

Dihydrostilbenoids (bibenzyls) are natural phenols formed from the dihydrostilbene (bibenzyl) backbone.

Eucomis bicolor, the variegated pineapple lily or just pineapple lily, is a bulbous species of flowering plant in the family Asparagaceae, subfamily Scilloideae, native to Southern Africa. The pale green, purple-margined flowers are arranged in a spike (raceme), topped by a "head" of green leaflike bracts. It is cultivated as an ornamental bulbous plant, although its flowers have an unpleasant smell, attractive to the main pollinators, flies.
2,4,7-trihydroxy-1,4-benzoxazin-3-one-glucoside 7-O-methyltransferase is an enzyme with systematic name S-adenosyl-L-methionine:(2R)-4,7-dihydroxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl β-D-glucopyranoside 7-O-methyltransferase. This enzyme catalyses the following chemical reaction
2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one 2-D-glucosyltransferase is an enzyme with systematic name UDP-alpha-D-glucose:2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one 2-beta-D-glucosyltransferase. This enzyme catalyses the following chemical reaction

Pseudoprospero is a genus of bulbous flowering plants in the family Asparagaceae, subfamily Scilloideae. The genus has a single species Pseudoprospero firmifolium, which is endemic to South Africa.
4-Hydroxy-7-methoxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl glucoside beta-D-glucosidase (EC 3.2.1.182, DIMBOAGlc hydrolase, DIMBOA glucosidase) is an enzyme with systematic name (2R)-4-hydroxy-7-methoxy-3-oxo-3,4-dihydro-2H-1,4-benzoxazin-2-yl beta-D-glucopyranoside beta-D-glucosidase. This enzyme catalyses the following chemical reaction

Cremastra appendiculata is an orchid species in the genus Cremastra. It is the type species of its genus.

Ledebouria floribunda is a species of flowering plant in the Asparagaceae family. It is a bulbous geophyte native to South Africa, Eswatini, and Lesotho.

Ledebouria revoluta, the south Indian squill, is a flowering plant species in the genus Ledebouria found in Southern Africa and India.

Albuca fastigiata is a plant species in the genus Albuca native to the Cape Provinces of South Africa, where it is used in ethnomedicine.

Eucomis montana is a plant species in the family Asparagaceae, subfamily Scilloideae, found in South Africa and Eswatini (Swaziland). When in flower in summer, the plant reaches a height of up to 45 cm, with a dense spike (raceme) of greenish flowers, topped by a "head" of green bracts.
















