
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body.

Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear weapons reprocessing. The storage and disposal of radioactive waste is regulated by government agencies in order to protect human health and the environment.

A beta particle, also called beta ray or beta radiation, is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β− decay and β+ decay, which produce electrons and positrons respectively.
A synthetic radioisotope is a radionuclide that is not found in nature: no natural process or mechanism exists which produces it, or it is so unstable that it decays away in a very short period of time. Examples include technetium-99 and promethium-146. Many of these are found in, and harvested from, spent nuclear fuel assemblies. Some must be manufactured in particle accelerators.
Ionizing radiation, including nuclear radiation, consists of subatomic particles or electromagnetic waves that have sufficient energy to ionize atoms or molecules by detaching electrons from them. Some particles can travel up to 99% of the speed of light, and the electromagnetic waves are on the high-energy portion of the electromagnetic spectrum.
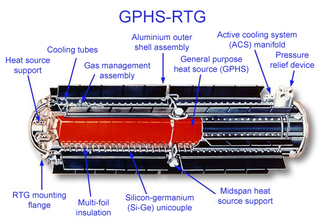
A radioisotope thermoelectric generator, sometimes referred to as a radioisotope power system (RPS), is a type of nuclear battery that uses an array of thermocouples to convert the heat released by the decay of a suitable radioactive material into electricity by the Seebeck effect. This type of generator has no moving parts and is ideal for deployment in remote and harsh environments for extended periods with no risk of parts wearing out or malfunctioning.

Nuclear chemistry is the sub-field of chemistry dealing with radioactivity, nuclear processes, and transformations in the nuclei of atoms, such as nuclear transmutation and nuclear properties.
Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation, and the means for achieving this". Exposure can be from a source of radiation external to the human body or due to internal irradiation caused by the ingestion of radioactive contamination.

Nuclear medicine, or nucleology, is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging is, in a sense, radiology done inside out, because it records radiation emitted from within the body rather than radiation that is transmitted through the body from external sources like X-ray generators. In addition, nuclear medicine scans differ from radiology, as the emphasis is not on imaging anatomy, but on the function. For such reason, it is called a physiological imaging modality. Single photon emission computed tomography (SPECT) and positron emission tomography (PET) scans are the two most common imaging modalities in nuclear medicine.
A radioactive tracer, radiotracer, or radioactive label is a synthetic derivative of a natural compound in which one or more atoms have been replaced by a radionuclide. By virtue of its radioactive decay, it can be used to explore the mechanism of chemical reactions by tracing the path that the radioisotope follows from reactants to products. Radiolabeling or radiotracing is thus the radioactive form of isotopic labeling. In biological contexts, experiments that use radioisotope tracers are sometimes called radioisotope feeding experiments.

Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases, where their presence is unintended or undesirable.
Phosphorus-32 (32P) is a radioactive isotope of phosphorus. The nucleus of phosphorus-32 contains 15 protons and 17 neutrons, one more neutron than the most common isotope of phosphorus, phosphorus-31. Phosphorus-32 only exists in small quantities on Earth as it has a short half-life of 14 days and so decays rapidly.

Iodine-131 is an important radioisotope of iodine discovered by Glenn Seaborg and John Livingood in 1938 at the University of California, Berkeley. It has a radioactive decay half-life of about eight days. It is associated with nuclear energy, medical diagnostic and treatment procedures, and natural gas production. It also plays a major role as a radioactive isotope present in nuclear fission products, and was a significant contributor to the health hazards from open-air atomic bomb testing in the 1950s, and from the Chernobyl disaster, as well as being a large fraction of the contamination hazard in the first weeks in the Fukushima nuclear crisis. This is because 131I is a major fission product of uranium and plutonium, comprising nearly 3% of the total products of fission. See fission product yield for a comparison with other radioactive fission products. 131I is also a major fission product of uranium-233, produced from thorium.

There are 37 known isotopes of iodine (53I) from 108I to 144I; all undergo radioactive decay except 127I, which is stable. Iodine is thus a monoisotopic element.

[18F]Fluorodeoxyglucose (INN), or fluorodeoxyglucose F 18, also commonly called fluorodeoxyglucose and abbreviated [18F]FDG, 2-[18F]FDG or FDG, is a radiopharmaceutical, specifically a radiotracer, used in the medical imaging modality positron emission tomography (PET). Chemically, it is 2-deoxy-2-[18F]fluoro-D-glucose, a glucose analog, with the positron-emitting radionuclide fluorine-18 substituted for the normal hydroxyl group at the C-2 position in the glucose molecule.
Iodine-125 (125I) is a radioisotope of iodine which has uses in biological assays, nuclear medicine imaging and in radiation therapy as brachytherapy to treat a number of conditions, including prostate cancer, uveal melanomas, and brain tumors. It is the second longest-lived radioisotope of iodine, after iodine-129.

This page discusses each of the main elements in the mixture of fission products produced by nuclear fission of the common nuclear fuels uranium and plutonium. The isotopes are listed by element, in order by atomic number.

This article compares the radioactivity release and decay from the Chernobyl disaster with various other events which involved a release of uncontrolled radioactivity.

A gamma ray, also known as gamma radiation (symbol
γ
), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically shorter than those of X-rays. With frequencies above 30 exahertz (3×1019 Hz) and wavelengths less than 10 picometers (1×10−11 m), gamma ray photons have the highest photon energy of any form of electromagnetic radiation. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900 while studying radiation emitted by radium. In 1903, Ernest Rutherford named this radiation gamma rays based on their relatively strong penetration of matter; in 1900, he had already named two less penetrating types of decay radiation (discovered by Henri Becquerel) alpha rays and beta rays in ascending order of penetrating power.

Radiopharmaceuticals, or medicinal radiocompounds, are a group of pharmaceutical drugs containing radioactive isotopes. Radiopharmaceuticals can be used as diagnostic and therapeutic agents. Radiopharmaceuticals emit radiation themselves, which is different from contrast media which absorb or alter external electromagnetism or ultrasound. Radiopharmacology is the branch of pharmacology that specializes in these agents.
















