Related Research Articles
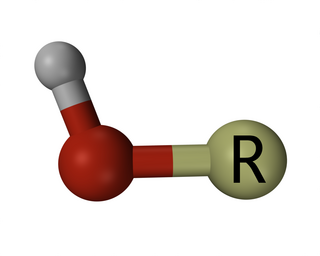
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula −OH and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy groups. Both the negatively charged anion HO−, called hydroxide, and the neutral radical HO·, known as the hydroxyl radical, consist of an unbonded hydroxy group.

Spectroscopy is the field of study that measures and interprets electromagnetic spectrum. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
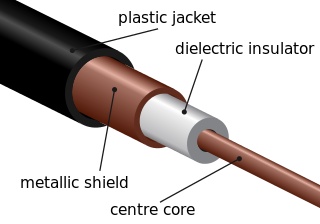
A transmission medium is a system or substance that can mediate the propagation of signals for the purposes of telecommunication. Signals are typically imposed on a wave of some kind suitable for the chosen medium. For example, data can modulate sound, and a transmission medium for sounds may be air, but solids and liquids may also act as the transmission medium. Vacuum or air constitutes a good transmission medium for electromagnetic waves such as light and radio waves. While a material substance is not required for electromagnetic waves to propagate, such waves are usually affected by the transmission media they pass through, for instance, by absorption or reflection or refraction at the interfaces between media. Technical devices can therefore be employed to transmit or guide waves. Thus, an optical fiber or a copper cable is used as transmission media.
Atomic, molecular, and optical physics (AMO) is the study of matter–matter and light–matter interactions, at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theory includes classical, semi-classical and quantum treatments. Typically, the theory and applications of emission, absorption, scattering of electromagnetic radiation (light) from excited atoms and molecules, analysis of spectroscopy, generation of lasers and masers, and the optical properties of matter in general, fall into these categories.
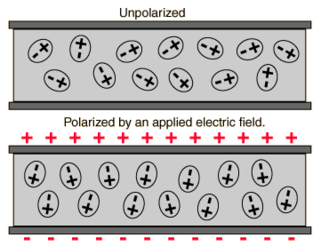
In electromagnetism, the absolute permittivity, often simply called permittivity and denoted by the Greek letter ε (epsilon), is a measure of the electric polarizability of a dielectric material. A material with high permittivity polarizes more in response to an applied electric field than a material with low permittivity, thereby storing more energy in the material. In electrostatics, the permittivity plays an important role in determining the capacitance of a capacitor.

Ionization is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules, electrons, positrons, protons, antiprotons and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected.
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate BO3−3, metaborate BO−2, or tetraborate B4O2−7; or any salt of such anions, such as sodium metaborate, Na+[BO2]− and borax (Na+)2[B4O7]2−. The name also refers to esters of such anions, such as trimethyl borate B(OCH3)3 but they are alkoxides.

Ultraviolet (UV) spectroscopy or ultraviolet–visible (UV–VIS) spectrophotometry refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum. Being relatively inexpensive and easily implemented, this methodology is widely used in diverse applied and fundamental applications. The only requirement is that the sample absorb in the UV-Vis region, i.e. be a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy. Parameters of interest, besides the wavelength of measurement, are absorbance (A) or transmittance (%T) or reflectance (%R), and its change with time.

Laser cooling includes several techniques where atoms, molecules, and small mechanical systems are cooled with laser light. The directed energy of lasers is often associated with heating materials, e.g. laser cutting, so it can be counterintuitive that laser cooling often results in sample temperatures approaching absolute zero. It is a routine step in many atomic physics experiments where the laser-cooled atoms are then subsequently manipulated and measured, or in technologies, such as atom-based quantum computing architectures. Laser cooling relies on the change in momentum when an object, such as an atom, absorbs and re-emits a photon. For example, if laser light illuminates a warm cloud of atoms from all directions and the laser's frequency is tuned below an atomic resonance, the atoms will be cooled. This common type of laser cooling relies on the Doppler effect where individual atoms will preferentially absorb laser light from the direction opposite to the atom's motion. The absorbed light is re-emitted by the atom in a random direction. After repeated emission and absorption of light the net effect on the cloud of atoms is that they will expand more slowly. The slower expansion reflects a decrease in the velocity distribution of the atoms, which corresponds to a lower temperature and therefore the atoms have been cooled. For an ensemble of particles, their thermodynamic temperature is proportional to the variance in their velocity, therefore the lower the distribution of velocities, the lower temperature of the particles.
In physics, atomic spectroscopy is the study of the electromagnetic radiation absorbed and emitted by atoms. Since unique elements have unique emission spectra, atomic spectroscopy is applied for determination of elemental compositions. It can be divided by atomization source or by the type of spectroscopy used. In the latter case, the main division is between optical and mass spectrometry. Mass spectrometry generally gives significantly better analytical performance, but is also significantly more complex. This complexity translates into higher purchase costs, higher operational costs, more operator training, and a greater number of components that can potentially fail. Because optical spectroscopy is often less expensive and has performance adequate for many tasks, it is far more common. Atomic absorption spectrometers are one of the most commonly sold and used analytical devices.

The hydroxyl radical, •HO, is the neutral form of the hydroxide ion (HO–). Hydroxyl radicals are highly reactive and consequently short-lived; however, they form an important part of radical chemistry. Most notably hydroxyl radicals are produced from the decomposition of hydroperoxides (ROOH) or, in atmospheric chemistry, by the reaction of excited atomic oxygen with water. It is also an important radical formed in radiation chemistry, since it leads to the formation of hydrogen peroxide and oxygen, which can enhance corrosion and stress corrosion cracking in coolant systems subjected to radioactive environments. Hydroxyl radicals are also produced during UV-light dissociation of H2O2 (suggested in 1879) and likely in Fenton chemistry, where trace amounts of reduced transition metals catalyze peroxide-mediated oxidations of organic compounds.

Electromagnetically induced transparency (EIT) is a coherent optical nonlinearity which renders a medium transparent within a narrow spectral range around an absorption line. Extreme dispersion is also created within this transparency "window" which leads to "slow light", described below. It is in essence a quantum interference effect that permits the propagation of light through an otherwise opaque atomic medium.

In physics, absorption of electromagnetic radiation is how matter takes up a photon's energy — and so transforms electromagnetic energy into internal energy of the absorber.

Holmium(III) oxide, or holmium oxide is a chemical compound of the rare-earth element holmium and oxygen with the formula Ho2O3. Together with dysprosium(III) oxide (Dy2O3), holmium oxide is one of the most powerfully paramagnetic substances known. The oxide, also called holmia, occurs as a component of the related erbium oxide mineral called erbia. Typically, the oxides of the trivalent lanthanides coexist in nature, and separation of these components requires specialized methods. Holmium oxide is used in making specialty colored glasses. Glass containing holmium oxide and holmium oxide solutions have a series of sharp optical absorption peaks in the visible spectral range. They are therefore traditionally used as a convenient calibration standard for optical spectrophotometers.

An optical fiber, or optical fibre, is a flexible glass or plastic fiber that can transmit light from one end to the other. Such fibers find wide usage in fiber-optic communications, where they permit transmission over longer distances and at higher bandwidths than electrical cables. Fibers are used instead of metal wires because signals travel along them with less loss and are immune to electromagnetic interference. Fibers are also used for illumination and imaging, and are often wrapped in bundles so they may be used to carry light into, or images out of confined spaces, as in the case of a fiberscope. Specially designed fibers are also used for a variety of other applications, such as fiber optic sensors and fiber lasers.
The refractive index of water at 20 °C for visible light is 1.33. The refractive index of normal ice is 1.31. In general, an index of refraction is a complex number with real and imaginary parts, where the latter indicates the strength of absorption loss at a particular wavelength. In the visible part of the electromagnetic spectrum, the imaginary part of the refractive index is very small. However, water and ice absorb in infrared and close the infrared atmospheric window, thereby contributing to the greenhouse effect.

The absorption of electromagnetic radiation by water depends on the state of the water.
The Gladstone–Dale relation is a mathematical relation used for optical analysis of liquids, the determination of composition from optical measurements. It can also be used to calculate the density of a liquid for use in fluid dynamics. The relation has also been used to calculate refractive index of glass and minerals in optical mineralogy.

Fluoride glass is a class of non-oxide optical glasses composed of fluorides of various metals. They can contain heavy metals such as zirconium, or be combined with lighter elements like aluminium and beryllium. These heavier elements cause the glass to have a transparency range extended into the infrared wavelength.
Photoelectrochemical processes are processes in photoelectrochemistry; they usually involve transforming light into other forms of energy. These processes apply to photochemistry, optically pumped lasers, sensitized solar cells, luminescence, and photochromism.
References
- ↑ "Hydroxyl Ion Absorption". Timbercon. Retrieved 16 September 2024.
- ↑ Wu, Lue; Gao, Maodong; Liu, Jin-Yu; Chen, Hao-Jing; Colburn, Kellan; Blauvelt, Henry A.; Vahala, Kerry J. (1 July 2023). "Hydroxyl ion absorption in on-chip high-Q resonators". Optics Letters. 48 (13): 3511. arXiv: 2303.17814 . doi:10.1364/OL.492067. ISSN 0146-9592.
![]() This article incorporates public domain material from Federal Standard 1037C. General Services Administration. Archived from the original on 22 January 2022.
This article incorporates public domain material from Federal Standard 1037C. General Services Administration. Archived from the original on 22 January 2022.